UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
________________
Form 10-K
|
(Mark One) |
|
☑ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934. |
|
|
For the fiscal year ended December 31, 2020 |
||
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934. |
|
|
For the transition period from ___ to _____ |
Commission file number: 1-16525
CVD EQUIPMENT CORPORATION
(Exact name of registrant as specified in its charter)
|
New York |
11-2621692 |
|
(State or Other Jurisdiction of |
(I.R.S. Employer Identification No.) |
|
355 South Technology Drive Central Islip, New York 11722 |
|
|
(Address including zip code of registrant’s Principal Executive Offices) |
|
(631) 981-7081
(Registrant’s Telephone Number, Including Area Code)
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
|
Common Stock, Par value $0.01 |
CVV |
NASDAQ Capital Market |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☑
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☑
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☑ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data file required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months/(or for such shorter period that the registrant was required to submit such files). Yes ☑ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer a smaller reporting company or an emerging growth company. See the definitions of "large accelerated filer,” "accelerated filer,” "smaller reporting company” and emerging growth company in Rule 12b-2 of the Exchange Act.
Large accelerated filer ☐ Accelerated filer ☐ Non-accelerated filer ☑ Smaller reporting company ☑ Emerging Growth Company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☑
State the aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant’s most recently completed second fiscal quarter: $20,045,343 at June 30, 2020.
Indicate the number of shares outstanding of each of the registrant’s classes of common stock, as of the latest practicable date: 6,681,781 shares of Common Stock, $0.01 par value at March 24, 2021.
DOCUMENTS INCORPORATED BY REFERENCE: None.
__________________________________________________________________________
PART I
INFORMATION CONCERNING FORWARD-LOOKING STATEMENTS
Except for historical information contained herein, this Annual Report on Form 10-K contains forward–looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended and Section 21E of the Securities Exchange Act of 1934, as amended. Readers are cautioned not to place undue reliance on forward-looking statements, as there can be no assurance that the plans, intentions or expectations upon which they are based will occur. These statements involve known and unknown risks and uncertainties that may cause our actual results or outcomes to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. These forward-looking statements are based on various factors and are derived utilizing numerous important assumptions and other important factors that could cause actual results to differ materially from those in the forward-looking statements. Important assumptions and other factors that could cause actual results to differ materially from those in the forward-looking statements, include, but are not limited to: competition in our existing and potential future product lines of business; our ability to obtain financing on acceptable terms if and when needed; uncertainty as to our future profitability, uncertainty as to the future profitability of acquired businesses or product lines, uncertainty as to any future expansion of the Company and the continued effect of the novel coronavirus (COVID-19) pandemic on our business and operations, and those of our customers, suppliers and other third parties. Other factors and assumptions not identified above were also involved in the derivation of these forward-looking statements and the failure of such assumptions to be realized as well as other factors may also cause actual results to differ materially from those projected. We assume no obligation to update these forward-looking statements to reflect actual results, changes in assumptions, or changes in other factors affecting such forward-looking statements. Past performance is no guaranty of future results.
Item 1. Description of Business.
The use of the words "CVD,” "we,” "us” or "our” refers to CVD Equipment Corporation, a New York corporation incorporated on October 13, 1982, and its wholly owned subsidiaries, CVD Materials Corporation (including its wholly owned subsidiaries CVD Tantaline ApS, and CVD MesoScribe Technologies Corporation) collectively "CVD Materials”), FAE Holdings 411519R LLC and 555 N Research Corporation except where the context otherwise requires.
We develop, design, manufacture and service a broad range of chemical vapor deposition, gas control and other state-of-the-art process equipment and solutions used in research & development and production of advanced materials and coatings. Our served markets extend from research to industrial applications. This equipment is used by our customers to research, design, and manufacture these materials or coatings for aerospace engine and structural components, medical devices such as implants, advanced semiconductor devices, solar cells, smart glass, carbon nanotubes, nanowires, LEDs, MEMS and other applications. Through CVD Materials and our Application Laboratory, we develop new material systems, provide material coating services, process development support and process startup assistance with the focus on enabling tomorrow’s technologiesTM.
Based on more than 39 years of experience, we use our capabilities in process development, engineering and manufacturing to transform new applications into leading-edge manufacturing solutions. This enables university, research and industrial scientists at the cutting edge of technology to develop next generation aerospace, medical, nano, LEDs, semiconductors and other electronic components. We develop, manufacture and provide equipment for research and production based on our proprietary designs. We have built a significant library of design expertise, know-how and innovative solutions to assist our customers in developing these intricate processes and to accelerate their commercialization. This library of equipment design solutions, along with our manufacturing and systems integration facilities, allows us to provide superior design, process and manufacturing solutions to our customers.
Our strategy is to target opportunities in the research, development and production equipment market, with a focus on high growth applications such as aerospace, carbon nanotubes, nanowires, medical, graphene, MEMS and LEDs. To expand our penetration into these growth markets, we have developed a line of proprietary standard products and custom systems. Historically, we manufactured products for research and development on an applications specific basis to meet an individual customer’s specific research requirements. Our proprietary systems leverage the technological expertise that we have developed through designing these custom systems onto a standardized basic core. This core is easily adapted through a broad array of available options to meet the diverse product and budgetary requirements of the research community. By manufacturing the basic core of these systems in higher volumes, we are able to reduce both the cost and delivery time for our systems. These systems, which we market and sell under the EasyTube® and CVD product lines, are sold to researchers at universities, research laboratories, and startup companies in the United States and throughout the world.
Sales of our proprietary standard systems, custom systems and process solutions have been driven by our installed customer base, which includes Fortune 500 companies. The strong performance and success of our products has historically driven repeat orders from existing customers as well as business from new customers. Furthermore, with our proprietary solutions and expanded focus on "accelerating the commercialization of tomorrow’s technologies”TM we have been developing a new customer base in addition to growing with our existing customers. We have generally gained new customers through our reputation in the markets and industries, limited print advertising and trade show attendance (which has been negatively impacted by COVID-19).
The core competencies we have developed in equipment and software design, as well as in systems manufacturing and process solutions, are used to engineer our finished products and to accelerate the commercialization path of our customer base. Our proprietary-real-time, software allows for rapid configuration, and provides our customers with powerful tools to understand, optimize and repeatedly control their processes. These factors significantly reduce cost, improve quality and reduce the time it takes from customer order to shipment of our products. Our Application Laboratory allows customers the option to bring up their process tools in our Application Laboratory and to work together with our scientists and engineers to optimize process performance.
Current Developments
Historically, we have derived substantially all of our revenues through our custom equipment business and our Stainless Design Concepts ("SDC”) gas management and chemical delivery control systems segment. The marketing, sale and manufacture of our products, requires a lengthy sales cycle ranging from several months to over more than one year before we can complete production and delivery. Also, demand for our equipment and related consumable products and services may be volatile as a result of sudden changes in market conditions, competition and other factors. This can and has resulted in substantial volatility in our revenue stream.
In order to address this sales volatility, we have attempted to diversify and expand our business into providing material products and services. This strategy included the development of our capabilities to provide materials coatings and surface treatments for targeted customer / market requirements (the "Material Business”). With this objective in mind, we acquired Tantaline in December 2016 and MesoScribe in October 2017. In order to facilitate these new lines of businesses, we also purchased a building to house both operating subsidiaries for $13,850,000. This 180,000 square foot building (the "555 Building”) was to house the Material Business in the United States and provide adequate space for the anticipated growth of these businesses. In addition, we also maintain a 130,000 square foot building (the "355 Building”), which houses the equipment products portion of our business as well as our corporate headquarters.
We have invested approximately $1.6 million, $2.7 million and $2.5 million during 2020, 2019 and 2018, respectively, in building improvements, machinery, and other expenses related primarily to the Materials Business.
The projected growth of the Materials Business has not met expectations. Although we have made substantial investments in facilities, equipment and acquisitions in furtherance of our strategy, the foregoing has proven to be a significant drain on our finances and our liquidity. Since 2018 revenues for the Materials Business have been $1,700,000 in 2018, $1,600,000 in 2019 and $2,300,000 in 2020, with operating losses, exclusive of a $3.6 million impairment charge, recorded in all years for a total loss of $2.5 million. These cumulative results are due to operating losses from the Tantaline operations offset by operating profits of $.5 million from the MesoScribe operations.
Our mortgage debt on the 355 Building and 555 Building, in the amount of $2.1 million and $9.3 million respectively, at December 31 2020, matures in March and December 2022, respectively.
In January 2021, our Board of Directors concluded that we needed a change in direction and new leadership to evaluate our business strategy and operations, and take timely actions to halt and reverse the declines of the past few years. As such, they appointed Emmanuel Lakios as President and Chief Executive Officer (previously our Vice-President- Sales and Marketing). We began an intensive analysis of our entire business and operations including the Materials Business. Based upon that analysis we believe our primary focus should be on the core equipment business and that the Materials Business strategy should be revised, with some of its current elements potentially minimized or ceased. Based upon this analysis, we are forecasting continued losses and negative cash flow for our Tantaline product line and as a consequence, we have implemented plans to eliminate further investment in our Tantaline product line, which will result in the avoidance of approximately $1.5-$2.0 million in additional costs. In addition, we have recorded an impairment charge of $3.6 million during the fourth quarter and year ended December 31, 2020. Based upon certain decisions and actions currently being reviewed, there may be additional costs to be incurred, inclusive of employee related and lease termination costs estimated at approximately $400,000.
In order to increase our liquidity and to provide necessary working capital to support our on-going business and operations, we have decided to sell the 555 Building in February 2021. We have determined the 555 Building is not needed for present and future business operations. We have concluded that any remaining elements of the Materials Business can be consolidated into the 355 Building, which we believe can accommodate any needs for our growth for the foreseeable future.
On March 29, 2021, the Company entered into an agreement with Steel K, LLC for the sale of its 555 Building. The purchase price is $24,360,000, and the closing of the sale is subject to the satisfaction or waiver of certain conditions to closing or contingencies. A portion of the sale proceeds would be used to satisfy the existing mortgage debt on the 555 Building in the approximate amount of $9.3 million at December 31, 2020, and for various costs related to the sale closing in an amount to be determined. Any excess proceeds will be used for general working capital purposes.
Business Developments
CVD Materials Segment:
On October 31, 2017, through our newly formed and wholly-owned subsidiary, CVD MesoScribe Technologies Corporation ("CVD MesoScribe Technologies”), we acquired substantially all of the operating assets and business of MesoScribe Technologies, Inc. ("MTI”). Formed in 2002, by a group from Stony Brook University, MTI established itself as a pioneer and leader in the direct deposition of thermal sensors, heaters, and instrumentation for harsh environments.
MTI specialized in materials processing using Direct Write MesoPlasma™ printing technology. This technology is an enabling additive manufacturing process whereby materials are printed onto conformal components in precise patterns. MTI has provided MesoPlasma™ printing services and products to its customers for use in aerospace, power generation, satellite, and defense markets, focusing on developing and manufacturing innovative products for advanced sensing, heating, and communication.
This acquisition provided CVD access to an additional materials deposition technology, a presence in new markets including a broader presence in the aerospace and defense markets, and additional end user applications. In addition, the proprietary MesoPlasma™ technology complimented our Tantaline® business which we acquired in the fourth quarter of 2016. The two technologies when combined provide a treatment and coating which can provide both corrosion and wear resistance. This was consistent with our strategic plan to leverage our equipment know-how, business infrastructure and proven ability to scale up new technologies, all offering high value-added materials, products, and services and was another step in our combined organic and acquisition growth strategy.
In 2017, to support the expected growth of CVD Materials and to relocate the California MesoScribe operations as well as Tantaline USA business on November 30, 2017, we purchased the premises located at 555 North Research Place, Central Islip, NY (the "Premises”). The purchase price of the Premises was $13,850,000, exclusive of closing costs. On November 30, 2017, the Company’s newly formed wholly-owned subsidiary, 555 N Research Corporation (the "Assignee”) and the Islip IDA, entered into a Fee and Leasehold Mortgage and Security Agreement (the "Loan”) with HSBC Bank USA, N.A. (the "Bank”) in the amount of $10,387,500, which was used to finance a portion of the purchase price to acquire the Premises. The Loan was evidenced by the certain Note, dated November 30, 2017 (the "Note”), by and between Assignee and the Bank, and secured by a certain Fee and Leasehold Mortgage and Security Agreement, dated November 30, 2017 (the "Mortgage”), as well as a collateral Assignment of Leases and Rents ("Assignment of Leases”).
The Loan is payable in sixty consecutive equal monthly installments of $62,481, including interest. The Loan bears interest for each Interest Period (as defined in the Note), at the fixed rate of 3.9148%. The maturity date for the Note is December 1, 2022.
As a condition of the Bank making the Loan, the Company was required to guaranty Assignee’s obligations under the Loan pursuant to that certain Unlimited Guaranty, dated November 30, 2017 (the "Guaranty”).
Our new management evaluated our business projections, the utilization of our facilities and ability to raise capital to maintain and ultimately grow our operations, which as a result, has caused us to initiate the consolidation of the 555 Building into the 355 Building operations, and to sell the 555 Building.
In January 2021, our Board of Directors concluded that we needed a change in direction and new leadership to evaluate our business strategy and operations, and take timely actions to halt and reverse the declines of the past few years. As such, they appointed Emmanuel Lakios as President and Chief Executive Officer (previously our Vice-President- Sales and Marketing). We began an intensive analysis of our entire business and operations including the Materials Business. Based upon that analysis we believe our primary focus should be on the core equipment business and that the Materials Business strategy should be revised, with some of its current elements potentially minimized or ceased. Based upon this analysis, we are forecasting continued losses and negative cash flow for our Tantaline product line and as a consequence, we have implemented plans to eliminate further investment in our Tantaline product line, which will result in the avoidance of approximately $1.5-$2.0 million in additional costs. In addition, we have recorded an impairment charge of $3.6 million in the fourth quarter and year ended December 31, 2020. Based upon certain decisions and actions currently being reviewed, there may be additional costs to be incurred, inclusive of employee related and lease termination costs estimated at approximately $400,000.
With the pending completion of the consolidation of facilities, we believe we have adequate manufacturing space to accommodate our capabilities of providing materials, coatings and surface treatments to meet our customers’ needs as well as improved capital utilization and facilities cost absorption. We continue to be positioned for the expansion of our carbon composites and electronic materials, MesoScribe Technologies and other product lines.
We invested approximately $1.6 million, $2.7 million and $2.5 million during 2020, 2019 and 2018, respectively, in building improvements, machinery, and other expenses related primarily to the operations of CVD Materials. The start up of MesoScribe™ operations have been completed and it was fully operational during 2020, since the move of its operations in the middle of 2019 from California. Since September 2019, MesoScribe™ has secured orders and contracts in excess of $2.0 million, and in early 2020 we have hired four employees including test and project management to support those orders and growth opportunities. Tantaline® US equipment and facilitation continued to be installed in 2020 and was in various stages of process testing, qualification and refinement at the end of the year.
With regard to Tantaline® our Demark facility provides both local and global deposition services, although the majority of the business has been in Europe. The 555 Building was contemplated to primarily support the US customer base as well as serve as our application and technology center.
With respect to our CVD MesoScribe Technologies subsidiary, we have completed the process of consolidating the manufacturing and development operations in Central Islip in 2019. This has resulted in a reduction of our operating expenses associated with no longer leasing space in California and has provided synergies with the Central Islip sales and marketing functions and place the operations near the local Thermal Spray Center of Excellence located at Stony Brook University.
Segments
CVD/First Nano supplies state-of-the-art chemical vapor deposition systems for use in the research, development and manufacturing of aerospace, medical components, semiconductors, LEDs, carbon nanotubes, nanowires, solar cells and a number of other industrial applications. We utilize our expertise in the design and manufacture of chemical vapor deposition systems to work with laboratory scientists to bring state-of-the-art processes from the research laboratory into production, as well as to provide production equipment and process solutions based on our designs. CVD/First Nano also operates our Application Laboratory where our personnel interact effectively with the scientists and engineers of our customer base. CVD/First Nano operates from our main facility in Central Islip, New York.
SDC designs and manufactures ultra-high purity gas and chemical delivery control systems for state-of-the-art semiconductor fabrication processes, solar cells, LEDs, carbon nanotubes, nanowires, and a number of industrial applications. Our SDC products are sold on either a stand-alone basis, or together with our CVD/First Nano systems. SDC operates from a 22,000 square foot facility fitted with Class 10 and Class 100 clean room manufacturing space located in Saugerties, New York.
CVD Materials Has several elements and product groups. These are the Tantaline® corrosion resistant surface treatment, the MesoScribe robust material direct write, the Electronic Materials for advance electronics and Carbon Composite products.
Tantaline® treatment is a diffusion bonded protective layer of tantalum formed by chemical vapor deposition on the surface of common materials. Tantalum is the most corrosion resistant metal commercially available. This surface layer provides protection against many of the most aggressive environments, including high temperature concentrated acid. Global sales and technical support is provided by our facility in Central Islip, New York with production provided from our European facility located in Nordborg, Denmark. Tantaline®. We continue to develop new Tantalum processes to improve the corrosion resistance of additional base material such as Nickel based alloys. In 2018, we announced that two patent applications were filed. With the recent change in new leadership in January 2021, we began an intensive analysis of our entire business and operations including the Materials Business. Based upon that analysis we believe our primary focus should be on the core equipment business and that the Materials Business strategy should be revised, with some of its current elements potentially minimized or ceased. Based upon this analysis, we are forecasting continued losses and negative cash flow for our Tantaline product line and as a consequence, we have implemented plans to eliminate further investment in our Tantaline product line, which will result in the avoidance of approximately $1.5-$2.0 million in additional costs. In addition, we have recorded an impairment charge of $3.6 million in the fourth quarter and year ended December 31, 2020. Based upon certain decisions and actions currently being reviewed, there may be additional costs to be incurred, inclusive of employee related and lease termination costs estimated at approximately $400,000.
MesoScribe Technologies provides MesoPlasma™ printing services and products (heaters, antennas, and sensors) to aerospace, satellite, power generation, defense, and other markets requiring high performance. MesoScribe Technologies operated from a 22,000 square foot facility located in Huntington Beach, CA, until May 2019. The relocation of MesoSccribe’s operations to Central Islip, New York was completed during the third quarter of 2019. The consolidation of our Materials Business into the 355 Building is underway and estimated to be completed mid-year 2021.
Carbon Composites
Our developments and opportunities for the Carbon composite business come from achievements in our applications laboratory. In the fourth quarter of 2018, we announced the development of a family of advanced Fluid Reactors based on our innovations in nanotechnology and chemical vapor deposition technology. The Fluid Reactor is enabled by a novel reactor core element which allows the efficient transfer of gases into and out of liquids. The market adoption of this technology could supplant existing hollow fiber membrane technology for applications including filtration and liquid gasification or degasification. One such application being investigated is blood oxygenation cartridges, known as Extra Corporeal Membrane Oxygenators, which are typically used during cardio pulmonary bypass (CPB) surgery and are essential for life support. CVD has a patent pending embodying this technology. While holding promise the technology is in the evaluation phase and is not expected to generate revenue in the near future. We continue to investigate other application end uses for the liquid-gas and liquid-liquid exchange technology. As to this time there is not a near term revenue stream projected.
The applications lab, along with the sales and marketing team are exploring other possible carbon based products and applications that can be made from this carbon nano tube, infiltrated carbon and carbon nano fiber technology.
Principal Equipment Products
Chemical Vapor Deposition/Infiltration - A process which passes a gaseous compound over or through pores of a substrate material surface that is heated to such a degree that the compound decomposes and deposits a desired layer onto and or into a substrate material. The process is accomplished by combining appropriate gases in a reaction chamber, of the kind produced by the Company, at elevated temperatures (typically 150-1,600° Celsius). Our chemical vapor deposition systems are complete and include all necessary instrumentation, subsystems and components and include state-of-the-art process control software. We provide both standard and application specified engineered products. Some of the standard systems we offer are for silicon, silicon-germanium, silicon dioxide, silicon nitride, polysilicon, liquid phase epitaxial, metalorganic chemical vapor deposition, carbon nanotubes, graphene, silicon nanowires, solar cell research and solar material quality control.
Chemical Vapor Deposition Systems - Used in a variety of models for laboratory research and production. All models are offered as standard with total system automation, a microprocessor control system by which the user can measure, predict and regulate gas flow, temperature, pressure and chemical reaction rates, thus controlling the process in order to enhance the quality of the materials produced. Our standard microprocessor control system is extremely versatile and capable of supporting the complete product line and most custom system requirements. These chemical vapor deposition systems are typically priced between $100,000 and $2,500,000, but can be significantly higher for plant size chemical vapor deposition systems.
Rapid Thermal Processing ("RTP”) - Used to heat semiconductor materials to elevated temperatures of up to 1,200° Celsius at rapid rates of up to 200° Celsius per second. Our RTP systems are offered for implant activation, oxidation, silicide formation and many other processes. We offer systems that can operate both at atmospheric or reduced pressures. Our RTP systems are priced up to $600,000.
Annealing, Diffusion and Low Pressure Chemical Vapor Deposition (LPCVD) Furnaces - Used for diffusion, oxidation, implant anneal, solder reflow, solar cell manufacturing and other processes. The systems are normally operated at atmospheric and/or reduced pressure with gaseous atmospheres related to the process. An optional feature of the system allows for the heating element to be moved away from the process chamber allowing the wafers to rapidly cool or be heated in a controlled environment. Our cascade temperature control system enables more precise control of the wafers. The systems are equipped with an automatic process controller, permitting automatic process sequencing and monitoring with safety alarm provisions. Our annealing and diffusion furnace systems are priced up to $900,000.
Ultra-high Purity Gas and Liquid Control Systems - Our standard and custom designed gas and liquid control systems, which encompass gas cylinder storage cabinets, custom gas and chemical delivery systems, gas and liquid valve manifold boxes and gas isolation boxes, provide safe storage and handling of pressurized gases and chemicals. Our system design allows for automatic or manual control from both a local and remote location. A customer order often includes multiple systems to outfit a facility and can total up to $1,000,000.
Principal Materials Products
Quartz-ware - All process equipment, especially systems in production/manufacturing environments, require routine maintenance, consumable and spare parts. One such spare part and consumable which is core to our technology offering is quartz hardware. We provide standard and custom fabricated quartz-ware used in our equipment and to a lesser extent for other customer tools. We also provide repair and replacement of existing quartz-ware. The business volume is favorably impacted by our CVD/First Nano systems being in production/manufacturing environments.
MesoPlasma™ Direct Write Printing: A materials deposition process that provides robust high definition instrumentation, fine feature patterns, and coatings onto conformal components. Powder materials are injected into a thermal plasma where they are rapidly heated and deposited onto the substrate or component. A 6-axis robotic system ensures pattern placement accuracy and manufacturing consistency. The versatility of the process enables a wide range of materials to be deposited including ceramic dielectrics, nickel-based sensor alloys, metallic conductors, precious metals, and protective coatings. Products include temperature sensors, heaters, antennas and patterns per customer specifications.
Tantaline® Corrosion Resistant Coating: Tantaline® treatment is provided as part of either a finished product or as a service applied to customer sourced components. These include valves, fittings, fasteners, vessels, bellows, and a wide range of custom designed items. The Tantaline® treatment drastically improves the corrosion resistance of these base stainless-steel parts extending the service life and increasing value in a wide range of applications.
Markets and Marketing
We serve multiple emerging and mature global markets including aerospace, defense, biomedical implants, microelectronic and micromechanical devices, semiconductor, universities and research centers. Due to the highly technical nature of our products, we believe it is essential to contact customers directly through our sales personnel and through a network of domestic and international independent sales representatives and distributors specializing in the type of equipment, products and services that we sell. In addition to our traditional customer base, we are now accessing new markets and new customers through MesoScribe,® and other components of our materials business. Our primary marketing activities include direct sales contacts, participation in trade shows (which were significantly impacted in 2020 by COVID-19) and our internet websites www.cvdequipment.com, cvdmaterialscorporation.com, www.stainlessdesign.com, www.firstnano.com, tantaline.com and www.MesoScribe.com.
Customers
We continue to work on expanding our product and service offerings. Our systems and products are used in research and in production applications. We market and sell primarily to aerospace/defense, electronic component manufacturers, institutions involved in electronic component research (such as universities, government and industrial laboratories) and to industries such as medical that require specialized coatings. We have both a domestic and international customer base with hundreds of installed systems.
Given the complexity of some of the systems we sell, revenue from a single customer in any one year can exceed 10.0% of our total sales. Two customers represented 30.5% in total and two customers represented 39.3%, respectively, of our annual revenues in fiscal years 2020 and 2019. We believe that our relationships with these customers are positive and may provide us with ongoing continuous sustainability for years to come, however the loss of a large customer would have to be replaced by others, and our inability to do so has had a material adverse effect on our business and financial condition.
For the year ended December 31, 2020, approximately $2.8 million or 16.8% of our revenues were generated by sales to customers outside the U.S., compared to approximately $4.2 million or 21.3% for the year ended December 31, 2019.
Warranties
Warranties on our equipment are typically for twelve months but can range up to twenty-four months from shipment with extended contracts. We furnish any warranties from original manufacturers of components used in our products. We provide service and support for our installed base of equipment with in-house field service personnel. Warranty costs, including those incurred in fiscal years 2020 and 2019, are and have been historically insignificant and expensed as incurred.
Competition
We can experience intense direct competition from both domestic and international competitors in all of our equipment segments. Our First Nano product line, targeted at universities and introductory sites, faces price pressure from lower value-added competitors. Our MesoScribe operations, which is in the adoption phase, faces barriers from established indirect competitors of existing solution providers. We are aware of other competitors that offer a substantial number of products and services comparable to ours. Many of our competitors (including customers who may elect to manufacture systems for internal use) have financial, marketing and other resources greater than ours. To date, we believe that each of our product and service segments has been able to compete favorably in markets that include these competitors, primarily on the basis of know-how, technical performance, quality, delivery, price and aftermarket support. We will continue to focus on products which serve both markets that are growing and where we have a technical and commercial competitive advantage.
CVD/First Nano competes with companies located in Asia, Europe and the US in the research market. These companies have limited support and safety and system design capabilities. For the academia market, we also compete with laboratory built systems. Our equipment for production applications competes with in-house design and engineering capability and the capacity to build their own equipment internally. Additionally, there are large, established companies who compete with us and pose a competitive risk in the market. Due to budgetary and funding constraints, many customers are price sensitive. We believe that our systems are among the most advanced available for the targeted market, and coupled with our vertical integration in engineering and manufacturing, we can be very competitive.
SDC’s gas management and chemical delivery control systems are among the most advanced available. We further believe that SDC is differentiated from our competitors through our intimate understanding of how the systems in which our products are incorporated are actually used in field applications. We have gained this understanding as a result of having designed and built complex process gas systems for CVD/First Nano as well as for a number of the world’s leading semiconductor, aerospace, medical, solar manufacturers, research laboratories and universities.
Sources of Supply
Many of the components used in producing our products are purchased from unrelated suppliers. We have OEM status with our suppliers but we are not obligated to purchase a pre-determined quantity. We are not dependent on a principal or major supplier and alternate suppliers are available. Subject to lead times, the components and raw materials we use in manufacturing our products are readily obtainable.
Currently we maintain a fully-equipped machine shop that we use to fabricate a significant portion of our metal components in-house, including the most complex designed parts of our equipment. Similarly, our quartz fabrication capability is sufficient to meet our quartz-ware needs.
Materials procured from the outside and/or manufactured internally undergo a rigorous quality control process to ensure that the parts meet or exceed our requirements and those of our customers. Upon final assembly, all equipment undergoes a final series of complete testing to ensure maximum product performance.
Backlog
As of December 31, 2020, our order backlog was approximately $5.7 million, compared to $8.9 million as of December 31, 2019, a decrease of $3.2 million. Contributing to and compounding this decline, is the negative effect the COVID-19 crisis has had in 2020 on the aerospace industry, which resulted from reduced travel and reduction of industry gas turbine engine sales. Aerospace sales have represented as much as 60% of our total revenue. We continue to work at diversifying our customer base away from any one customer as we focus on new opportunities with new and existing customers within our existing marketplaces and in new applications, including the start-up of the CVD Materials operations. The timing for completion of backlog varies depending on the product mix and can be as long as two years or as short as 30-60 days. Order backlog is usually a reasonable management tool to indicate expected revenues, however, it does not provide an assurance of future achievement or profits as order cancellations or delays are possible.
Intellectual Property
Our success is dependent, in part upon our proprietary technology and other proprietary rights. We have historically protected our proprietary information and intellectual property such as design specifications, blueprints, technical processes and employee know-how through the use of non-disclosure agreements. In addition, where we deem appropriate, we have, and will continue to file for patent protection of our proprietary technology that we believe has the potential to be incorporated into our products and sold to multiple customers. We also maintain and/or assert rights in certain trademarks relating to certain of our products and product lines, and claim copyright protection for certain proprietary software and documentation.
In the fourth quarter of 2018, we announced the development of a family of advanced Fluid Reactors based on our innovations in nanotechnology and chemical vapor deposition technology. The Fluid Reactor is enabled by a novel reactor core element which allows the efficient transfer of gases into and out of liquids. The market adoption of this technology could supplant existing hollow fiber membrane technology for applications including filtration and liquid gasification or degasification. One such application is blood oxygenation cartridges, known as Extra Corporeal Membrane Oxygenators, which are typically used during cardio pulmonary bypass (CPB) surgery and are essential for life support. CVD has a patent pending embodying this technology. CVD is continuing development of its Fluid Reactor technology and during 2019 filed two related US Provisional Patent Applications.
While patent, copyright and trademark protections for our intellectual property are important to different degrees for our various products and solutions, we believe our future success in highly dynamic markets is most dependent upon the technical competence and creative skills of our personnel and our ability to accelerate the commercialization of next generation intellectual properties. We attempt to protect our trade secrets and other proprietary information through non-disclosure agreements with our customers, suppliers, employees and consultants and other security measures.
Research and Development
The university research community is at the forefront of nanotechnology research, and we are focused on providing state-of-the-art systems to this market that will help bridge the gap between pioneering research and marketable products. Our Application Laboratory, together with a number of leading universities and startup companies with whom we partner from time to time, conducts cutting-edge research on the growth and infiltration of carbon nanotubes, graphene and nanowires as well as on selected aerospace manufacturing processes. The results of this research could have far reaching implications concerning the use and manufacture of carbon nanotubes, graphene, nanowires and aerospace coatings for many markets. Our intention is that together with leading edge universities and start-up companies and major aerospace/defense companies, we will leverage our collective expertise in this field, which will allow us to capitalize on commercial opportunities in the future.
In 2020, we incurred approximately $373,000 in research and development expenses as compared to $598,000 in 2019.
Government Regulation
We are subject to a variety of federal, state and local government regulations, such as environmental, labor and export control. We believe that we have obtained all necessary permits to operate our business and that we are in material compliance with all laws and regulations applicable to us. These regulations change on an ongoing basis and the effect of the changes could materially impact our business in certain technology areas and regions.
Insurance
Our products are used in our customers’ manufacturing processes which in some cases contain explosive, flammable, corrosive and toxic gases. There are potential exposures to personal injury as well as property damage, particularly if operated without regard to the design limits of the systems and components. Additionally, the end products of some of our customers are used in areas such as aerospace and high-tech devices where safety is of great concern. Management reviews its insurance coverage on an annual basis or more frequently if appropriate and we believe we have the types and amounts of insurance coverage that are sufficient for our business.
Employees
At December 31, 2020, we had 130 employees. We had 62 employees in manufacturing, 28 in engineering (including research and development and efforts related to product improvement) 4 in field service, 9 in sales and marketing and 27 in general management, maintenance and administration, compared to 172 employees as of December 31, 2019. None of our employees were subject to a collective bargaining agreement. We generally consider our employee relations to be good.
The implementation of our business strategy largely depends on our ability to hire, train, and retain qualified and diverse professionals, and we must emphasize employee development and training in order to do so. We are committed to identifying and developing the talents of our next generation of managers and intend to establish a strong succession planning program for our critical positions, including internships for technical and engineering resources from local universities. Moreover, a key strategic focus of our new management team is to foster and maintain a strong and healthy culture, where collaboration to achieve results and focus on the success of our customers and shareholders is paramount.
Employee Compensation
In addition, our new management is undertaking a review of our compensation programs to better align the compensation of our employees with our performance, and to provide the proper short-term and long-term incentives to attract, retain and motivate them to achieve superior results. We believe we must offer wages that are competitive and consistent with employee positions, skill levels, experience and knowledge, and in order to do so we may work with a nationally recognized outside compensation and benefits consulting firm to independently evaluate the effectiveness of our executive and non-executive compensation and benefit programs and to provide benchmarking against our peers within our industry.
Employee Safety
The health and safety of our employees and partners is our highest priority, and this is consistent with our operating philosophy. We maintain strong environmental, health and safety protocols that focus on implementing policies and training programs, as well as performing self audits to ensure our colleagues and partners leave the workplace safely on a daily basis. Our safety focus is also evident in our response to the COVID-19 pandemic, which included:
|
● |
adding work from home flexibility; |
|
● |
adjusting attendance policy is to encourage those who are sick to stay home; |
|
● |
increase in cleaning protocols; |
|
● |
establishing proper physical distancing procedures for employees who need to be on site; |
|
● |
providing additional personal protective equipment and cleaning supplies; |
|
● |
requiring masks to be worn at all locations, where allowed or required by local law; |
|
● |
limiting all domestic and international nonessential travel for all employees; and |
|
● |
implementing protocols to address actual and suspected COVID-19 cases and potential exposure. |
Item 1A. Risk Factors
In addition to the other information set forth in this Annual Report on Form 10-K, our shareholders should carefully consider the risk factors described below. The risks set forth below may not be the only risk factors relating to the Company. Any of these factors, many of which are beyond our control, could materially adversely affect our business, financial condition, operating results, cash flow and stock price.
We have significant investments in our CVD Materials segment, which could be met with further delays and ongoing losses that could further materially and adversely impact our financial results and cash flow.
In 2017, to support the expected growth of our CVD Materials segment and to house the US based business, on November 30, 2017, we purchased the 555 Building located at 555 North Research Place, Central Islip, NY (the "Premises”). The purchase price of the building was $13,850,000, exclusive of closing costs. We have monthly principal and interest payments of $62,481 and have invested $1.6 million, $2.7 million and $2.5 million during 2020, 2019 and 2018, respectively, in building improvements, machinery, and other expenses related primarily to CVD Materials. Since September 2019, MesoScribe™ has secured orders of in excess of $2 million, and in early 2020 we have hired four employees including test and project management to support those orders and growth opportunities. Tantaline® US equipment continued to be installed in 2020 and are in various stages of process testing and refinement.
The projected growth of the Materials Business has not met expectations. Although we have made substantial investments in facilities, equipment and acquisitions in furtherance of our strategy, the foregoing has proven to be a significant drain on our finances and our liquidity. Since 2018 revenues for the Materials Business have been $1,700,000 in 2018, $1,600,000 in 2019 and $2,300,000 in 2020, with operating losses recorded in all years for a total loss of $2.5 million, exclusive of a $3.6 million impairment charge. These cumulative results are due to operating losses from the Tantaline operations offset by operating profits of $.5 million from the MesoScribe operations.
In January 2021, our Board of Directors concluded that we needed a change in direction and new leadership to evaluate our business strategy and operations, and take timely actions to halt and reverse the declines of the past few years. As such, they appointed Emmanuel Lakios as President and Chief Executive Officer (previously our Vice-President- Sales and Marketing). We began an intensive analysis of our entire business and operations including the Materials Business. Based upon that analysis we believe our primary focus should be on the core equipment business and that the Materials Business strategy should be revised, with some of its current elements potentially minimized or ceased. Based upon this analysis, we are forecasting continued losses and negative cash flow for our Tantaline product line and as a consequence, we have implemented plans to eliminate further investment in our Tantaline product line, which will result in the avoidance of approximately $1.5-$2.0 million in additional costs. In addition, we have recorded an impairment charge of $3.6 million in the fourth quarter and year ended December 31, 2020. Based upon certain decisions and actions currently being reviewed, there may be additional costs to be incurred, inclusive of employee related and lease termination costs estimated at approximately $400,000.
In order to increase our liquidity and to provide necessary working capital to support our on-going business and operations, we have decided to sell the 555 Building. We have determined the 555 Building is not needed for present and future business operations. We have concluded that any remaining elements of the Materials Business can be consolidated into the 355 Building, which we believe can accommodate any needs for our growth for the foreseeable future.
We might require additional financing.
Our overall revenues have declined from $41.1 million in 2017 to $16.9 million in 2020. Cumulative operating losses for the last three years (2018-2020) totaled ($18.1 million), which are comprised of 2018 ($5.3 million), 2019 ($5.0 million) and 2020 ($7.8 million). As a result of these continuing losses, and the investments in the Materials Business, our cash balances have declined from $21.7 million at December 31, 2016 to $7.7 million as of December 31, 2020, and liquidity has been strained. Contributing to and compounding this decline, is the negative effect the COVID-19 crisis has had in 2020 on the aerospace industry, which resulted from reduced travel and reduction of industry gas turbine engine sales. Aerospace sales have represented as much as 60% of our total revenue.
Our mortgage debt on the 355 Building and 555 Building, in the amount of $2.1 million and $9.3 million respectively, at December 31 2020, matures in March and December 2022, respectively.
In January 2021, our Board of Directors concluded that we needed a change in direction and new leadership to evaluate our business strategy and operations, and take timely actions to halt and reverse the declines of the past few years. As such, they appointed Emmanuel Lakios as President and Chief Executive Officer (previously our Vice-President- Sales and Marketing). We began an intensive analysis of our entire business and operations including the Materials Business. Based upon that analysis we believe our primary focus should be on the core equipment business and that the Materials Business strategy should be revised, with some of its current elements potentially minimized or ceased. Based upon this analysis, we are forecasting continued losses and negative cash flow for our Tantaline product line and as a consequence, we have implemented plans to eliminate further investment in our Tantaline product line, which will result in the avoidance of approximately $1.5-$2.0 million in additional costs. In addition, we have recorded an impairment charge of $3.6 million in the fourth quarter and year ended December 31, 2020. Based upon certain decisions and actions currently being reviewed, there may be additional costs to be incurred, inclusive of employee related and lease termination costs estimated at approximately $400,000.
In order to increase our liquidity and to provide necessary working capital to support our on-going business and operations, we have decided to sell the 555 Building. We have determined the 555 Building is not needed for present and future business operations. We have concluded that any remaining elements of the Materials Business can be consolidated into the 355 Building, which we believe can accommodate any needs for our growth for the foreseeable future.
Our continuing operating losses and decline in revenues may make it difficult for us to obtain financing on commercially reasonable terms, if at all. If any financing is not available if and when required on commercially reasonable terms, if at all, or, even if available and we issue additional common stock, it may materially dilute the ownership interests of the then existing shareholders.
A pandemic, epidemic or outbreak of an infectious disease such as COVID-19 in the United States or worldwide has adversely affected our business.
Our operations expose us to risks associated with pandemics, epidemics or other public health emergencies, such as the recent outbreak of coronavirus disease (COVID-19) which has spread from China to the rest of the world. Outbreaks such as these have resulted, and can continue to result, in governments around the world implementing increasingly stringent measures to help control the spread, including quarantines, "shelter in place" and "stay at home" orders, travel restrictions, business curtailments, school closures, and other measures. These actions with respect to the COVID-19 outbreak have negatively impacted, and could continue to have negative impacts on, our operations, supply chain, transportation networks, customers and employees. The COVID-19 outbreak has materially and adversely affected, and any continuing economic downturn as a result of this pandemic could continue to adversely affect, demand for our products, and negatively impact our business or results of operations through the temporary closure of our operating locations or those of our customers or suppliers. In particular, the aerospace sector, for which we rely on a significant part of our business, has been faced with significant reductions to its business due to lack of air travel.
Since the end of our first quarter 2020, we have experienced the impacts of COVID-19 on our markets and operations which have included significant decreases in demand, supply chain disruptions, and logistics constraints. Given government mandates and concerns over employee safety, some of our production facilities were closed or significantly slowed production during the end of the first quarter 2020 and into the second quarter 2020. The extent to which COVID-19 has and may continue to adversely impact our business depends on future developments, which are highly uncertain and unpredictable, including new information concerning the severity of the outbreak and the effectiveness of actions globally to contain or mitigate its effects. While we expect this matter to materially and adversely impact our financial results, the current level of uncertainty over the economic and operational impacts of COVID-19 means the related financial impact cannot be reasonably estimated at this time.
We have made investments in our proprietary technologies. If third parties violate our proprietary rights, or accuse us of infringing upon their proprietary rights, such events could result in a loss of value of some of our intellectual property or costly litigation.
Our success is dependent in part on our technologies and our other proprietary rights. We believe that while patents can be useful and may be utilized by us in the future, they are not always necessary or feasible to protect our intellectual property. The process of seeking patent protection is lengthy and expensive, and we cannot be certain that applications will actually result in issued patents or that issued patents will be of sufficient scope or strength to provide meaningful protection or commercial advantage to us. In addition to patent protection, we have also historically protected our proprietary information and intellectual property such as design specifications, blueprints, technical processes and employee know-how, by limiting access to this confidential information and trade secrets and through the use of non-disclosure agreements. Other companies and individuals, including our larger competitors, may develop technologies that are similar or superior to our technology, or design around the intellectual property that we own or license. Our failure to adequately protect our intellectual property, could result in the reduction or extinguishment of our rights to such intellectual property. We also assert rights to certain trademarks relating to certain of our products and product lines. We have not filed trademark applications to protect such marks with any governmental agency, including, but not limited to the U.S. Patent and Trademark Office. We claim copyright protection for certain proprietary software and documentation, but we have not filed any copyright applications with the U.S. Copyright Office in connection with those works. As a result, we can give no assurance that our trademarks and copyrights will be upheld or successfully deter infringement by third parties.
While patent, copyright and trademark protection for our intellectual property may be important, we believe our future success in highly dynamic markets is most dependent upon the technical competence and creative skills of our personnel. We attempt to protect our trade secrets and other proprietary information through confidentiality agreements with our customers, suppliers, employees and consultants, and through other internal security measures. However, these employees, consultants and third parties may breach these agreements, and we may not have adequate remedies for wrongdoing. In addition, the laws of certain territories in which we sell our products may not protect our intellectual property rights to the same extent as do the laws of the United States.
Occasionally, we may receive communications from other parties asserting the existence of patent rights or other intellectual property rights that they believe cover certain of our products, processes, technologies or information. In addition, it is possible we could have a dispute with a customer on intellectual property utilized in their equipment. If such cases arise, we will evaluate our position and consider the available alternatives, which may include seeking licenses to use the technology in question on commercially reasonable terms, developing new alternative technology or defending our position. Nevertheless, we cannot ensure that we will be able to obtain licenses, or, if we are able to obtain licenses, that related terms will be acceptable, or that litigation or other administrative proceedings will not occur. Defending our intellectual property rights through litigation could be very costly. If we are not able to negotiate the necessary licenses on commercially reasonable terms or successfully defend our position, our ability to utilize such intellectual property could substantially inhibit our access to certain markets and our ability to compete in these markets which could have a material adverse effect on our financial position and results of operations.
We have a highly concentrated customer base so that changes in ordering patterns, delays or order cancellations could have a material adverse effect on our business and results of operations.
Two customers represented 30.5% in total and two customers represented 39.3%, respectively, of our annual revenues in fiscal years 2020 and 2019. We believe that our relationships with these customers are positive and may provide us with ongoing continuous sustainability for years to come, however the loss of a large customer would have to be replaced by others, and our inability to do so may have a material adverse effect on our business and financial condition. We expect that contracts or orders from a relatively limited number of customers will continue to account for a substantial portion of our business. The mix and type of customers, and sales to any single customer, may vary significantly from quarter to quarter and from year to year. If any of our significant customers do not place orders, or they substantially reduce, delay or cancel orders, we may not be able to replace the business in a timely manner or at all, which can and has had a material adverse effect on our results of operations and financial condition. Major customers may also seek, and on occasion receive, pricing, payment, intellectual property-related, or other commercial terms that are less favorable to us and can hurt our competitive position.
Our lengthy and variable sales cycle makes it difficult to predict our financial results.
The marketing, sale and manufacture of our products, often requires a lengthy sales cycle ranging from several months to over one year before we can complete production and delivery. The lengthy sales cycle makes forecasting the volume and timing of sales difficult, and raises additional risks that customers may cancel or decide not to enter into contracts. The length of the sales cycle depends on the size and complexity of the project, the customer’s in-depth evaluation of our products, and, in some cases, the protracted nature of a bidding process.
Because a significant portion of our operating expenses are fixed, we have and may continue to incur substantial expense before we earn associated revenue. If customer cancellations occur, they could result in the loss of anticipated sales without allowing us sufficient time to reduce our operating expenses.
We have and may continue to be required to take additional impairment charges on assets.
We are required to assess goodwill and indefinite-lived intangible assets at least annually for impairment, or on an interim basis, whenever certain events occur or circumstances change, such as an adverse change in business climate or a decline in the overall industry, that would more likely than not reduce the fair value below its carrying amount. We are also required to test our long-lived assets, including acquired intangible assets and property, plant and equipment, for recoverability and impairment whenever there are indicators or impairment, such as an adverse change in business climate.
As part of our long-term strategy, we have pursued acquisitions of other companies or assets, such as our acquisitions of assets owned by Tantaline ApS and MesoScribe Technologies, Inc. and may pursue future acquisitions of other companies or assets which could potentially increase our assets. Adverse changes in business conditions could materially impact our estimates of future operations and result in impairment charges to these assets. If our assets were impaired, our financial condition and results of operations could be materially and adversely affected.
Following our recent change in management, we began an intensive analysis of our entire business and operations including the Materials Business. Based upon that analysis we believe our primary focus should be on the core equipment business and that the Materials Business strategy should be revised, with some of its current elements potentially minimized or ceased. Based upon this analysis, we are forecasting continued losses and negative cash flow for our Tantaline product line and as a consequence, we have implemented plans to eliminate further investment in our Tantaline product line, which will result in the avoidance of approximately $1.5-$2.0 million in additional costs. In addition, we have recorded an impairment charge of $3.6 million in the fourth quarter and year ended December 31, 2020. Based upon certain decisions and actions currently being reviewed, there may be additional costs to be incurred, inclusive of employee related and lease termination costs estimated at approximately $400,000.
Our success is highly dependent on the technical, sales, marketing and managerial contributions of key individuals, including our newly appointed Chief Executive Officer and President, and we may be unable to retain these individuals or recruit others.
We depend on our senior executives including our newly appointed Chief Executive Officer and President, and certain key managers as well as, engineering, research and development, sales, marketing and manufacturing personnel, who are critical to our business. We do not have long-term employment agreements with our key employees. Furthermore, larger competitors may be able to offer more generous compensation packages to our executives and key employees, and therefore we risk losing key personnel to those competitors. If we were to lose the services of any of our key personnel, our engineering, product development, manufacturing and sales efforts could be slowed. We may also incur increased operating expenses, and be required to divert the attention of our senior executives to search for their replacements. The integration of any new personnel could disrupt our ongoing operations.
Acquisitions can result in an increase in our operating costs, divert management’s attention away from other operational matters and expose us to other associated risks.
We continually evaluate potential acquisitions of businesses and technologies, and we consider targeted acquisitions that expand our core competencies to be an important part of our future growth strategy. In the past, we have made acquisitions of other businesses with synergistic products, services and technologies, and plan to continue to do so in the future.
Acquisitions involve numerous risks, which include but are not limited to:
|
● |
difficulties and increased costs in connection with the integration of the personnel, operations, technologies, services and products of the acquired companies into our existing facilities and operations; |
|
● |
diversion of management’s attention from other operational matters; |
|
● |
failure to commercialize the acquired technology; |
|
● |
the potential loss of key employees of the acquired companies; |
|
● |
lack of synergy, or inability to realize expected synergies, resulting from the acquisitions; |
|
● |
the risk that the issuance of our common stock, if any, in an acquisition or merger could be dilutive to our shareholders; |
|
● |
the inability to obtain and protect intellectual property rights in key technologies and |
|
● |
the acquired assets becoming impaired as a result of technological advancements or worse-than-expected performance of the acquired assets. |
If demand declines for chemical vapor deposition, gas control and related equipment, or for carbon nanotube and nanowire deposition systems, our financial position and results of operations could be materially adversely affected.
Our products are utilized to develop and manufacture materials and coatings for industrial and research applications that are used in numerous markets including but not limited to aerospace, medical, solar, nano and advanced electronic components. A significant part of our growth strategy involves continued expansion of the sales of our products for industrial as well as research and development purposes by companies, universities and government-funded research laboratories. The availability of funds for these purposes may be subject to budgetary and political restrictions, as well as cost-cutting measures by manufacturers in the markets in which we operate.
If the availability of funds or the demand for capital equipment in the markets in which we operate declines, the demand for our products would also decline and our financial position and results of operations could be harmed.
The conditions of the markets in which we operate are volatile. The demand for our products and the profitability of our products can change significantly from period to period as a result of numerous factors.
The industries in which we operate are characterized by ongoing changes, including:
|
● |
the availability of funds for research and development; |
|
● |
global and regional economic conditions; |
|
● |
governmental budgetary and political constraints; |
|
● |
changes in the capacity utilization and production volume for research and industrial applications in the markets in which we operate; |
|
● |
the profitability and capital resources of manufacturers in the markets in which we operate; and |
|
● |
changes in technology. |
For these and other reasons, our results of operations for past periods may not necessarily be indicative of future operating results.
Volatile and cyclical demand for our products may make it difficult for us to accurately budget our expense levels, which are based in part on our projections of future revenues.
Demand for our equipment and related consumable products may be volatile as a result of sudden changes in supply and demand, and other factors in the manufacturing process. Our orders tend to be more volatile than our revenue, as any change in demand is reflected immediately in orders booked, which are net of cancellations, while revenue, tends to be recognized over multiple quarters as a result of procurement and production lead times, and the deferral of certain revenue under our revenue recognition policies. The fiscal period in which we are able to recognize revenue is also at times subject to the length of time that our customers require to evaluate the performance of our equipment. This could cause our quarterly operating results to fluctuate.
When cyclical fluctuations result in lower than expected revenue levels, operating results have been and may continue to be materially adversely affected and cost reduction measures have been and may continue to be necessary for us to remain competitive and financially sound. During a down cycle, we must be able to make timely adjustments to our cost and expense structure to correspond to the prevailing market conditions. In addition, during periods of rapid growth, we must be able to increase manufacturing capacity and the number of our personnel to meet customer demand, which may require additional liquidity. We can provide no assurance, that these objectives can be met in a timely manner in response to changes within the industry cycles in which we operate. If we fail to respond to these cyclical changes, our business could be seriously harmed. In 2018, 2019 and 2020, we reported pre-tax income (loss) of ($5,558,000), ($4,914,000) and ($7,602,000), respectively.
We do not have long-term volume production contracts with our customers, and we do not control the timing or volume of orders placed by our customers. Whether and to what extent our customers place orders for any specific products, and the mix and quantities of products included in those orders are factors beyond our control. Insufficient orders would result in under-utilization of our manufacturing facilities and infrastructure, and will negatively affect our financial position and results of operations.
We face significant competition and we are relatively small in size and have fewer resources in comparison with many of our competitors.
We face significant competition throughout the world, which may increase as certain markets in which we operate continue to evolve. Our future performance depends, in part, upon our ability to continue to compete successfully worldwide. Some of our competitors are diversified companies that have substantially greater financial resources and more extensive research, engineering, manufacturing, marketing and customer service and support capabilities than we can provide. We face competition from companies whose strategy is to provide a broad array of products, some of which compete with the products and services that we offer, as well as companies, universities and research laboratories that have the capacity to design and build their own equipment internally. These competitors may bundle their products and services in a manner that may discourage customers from purchasing our products. In addition, we face competition from smaller emerging processing equipment companies, whose strategy is to provide a portion of the products and services that we offer at often lower prices than ours, using innovative technology to sell products into specialized markets. Loss of competitive position could impair our prices, customer orders, revenue, gross margin and market share, any of which would negatively affect our financial position and results of operations. Our failure to compete successfully with these other companies would seriously harm our business. There is a risk that larger, better financed competitors will develop and market more advanced products than those we currently offer, or that competitors with greater financial resources may decrease prices, thereby putting us under financial pressure.
The health and environmental effects of nanotechnology are unknown, and this uncertainty could adversely affect the expansion of our business.
The health and environmental effects of nanotechnology are unknown. There is no scientific agreement on the health effects of nanomaterials in general and carbon nanotubes, in particular, but some scientists believe that in some cases, nanomaterials may be hazardous to an individual’s health or to the environment.
The science of nanotechnology is based on arranging atoms in such a way as to modify or build materials not made in nature; therefore, the effects are unknown. Future research into the effects of nanomaterials in general, and carbon nanotubes in particular, on health and environmental issues, may have an adverse effect on products incorporating nanotechnology. Since part of our growth strategy is based on sales of research equipment for the production of carbon nanotubes and the sale of such materials, the determination that these materials are harmful could adversely affect the expansion of our business.
We may experience increasing price pressure.
Our historical business strategy for many of our products has focused on product performance and customer service rather than on price. As a result of budgetary constraints, many of our customers are extremely price sensitive when purchasing capital equipment. If we are unable to obtain prices that allow us to continue to compete on the basis of product performance and customer service, our profit margins will be reduced.
We may not be able to keep pace with the rapid change in the technology we use in our products.
We believe that our continued success in the markets in which we operate depends, in part, on our ability to continually improve existing technologies and to develop and manufacture new products and product enhancements on a timely and cost-effective basis. We must be able to introduce these products and product enhancements into the market in a timely manner, in response to customer’s demands for higher-performance research and assembly equipment, customized to address rapid technological advances in capital equipment designs.
Technological innovations are inherently complex, and require long development cycles and appropriate professional staffing. Our future business success depends on our ability to develop and introduce new products, or new uses for existing products, that successfully address changing customer needs. Our success also depends on our ability to achieve market acceptance of our new products. In order to maintain our success in the marketplace, we may have to substantially increase our expenditures on research and development. If we do not develop and introduce new products, technologies or uses for existing products in a timely manner and continually find ways to reduce the cost of developing and producing them in response to changing market conditions or customer requirements, our business could be seriously harmed.
Manufacturing interruptions or delays could affect our ability to meet customer demand and lead to higher costs, while the failure to estimate customer demand accurately could result in excess or obsolete inventory.
Our business depends on timely supply of equipment, services and related products that meet the rapidly changing technical and volume requirements of our customers. Some key parts to our products are subject to long lead-times and/or obtainable only from a single supplier or limited group of suppliers. Cyclical industry conditions and the volatility of demand for manufacturing equipment increase capital, technical, operational and other risks for us and for companies throughout our supply chain. Further, these conditions may cause some suppliers to scale back operations, exit businesses, merge with other companies, or file for bankruptcy protection and possibly cease operations.
We may also experience significant interruptions of our manufacturing operations, delays in our ability to deliver products or services, increased costs or customer order cancellations as a result of:
|
● |
The failure or inability of suppliers to timely deliver sufficient quantities of quality parts on a cost-effective basis; |
|
● |
Volatility in the availability and cost of materials, including rare earth elements; |
|
● |
Difficulties or delays in obtaining required import or export approvals; |
|
● |
Information technology or infrastructure failures; and |
|
● |
Natural disasters or other events beyond our control (such as earthquakes, floods or storms, regional economic downturns, pandemics, social unrest, political instability, terrorism, or acts of war). |
|
● |
The effects of COVID-19 on our employees, suppliers and other third-parties upon which we rely. |
For example, we may source certain materials used in the development and manufacture of our products from regions affected by the COVID-19, which could make access to our supply chain difficult and could affect our business. If a supplier fails to meet our requirements concerning quality, cost, socially-responsible business practices, or other performance factors, we may transfer our business to alternative sources, which could entail manufacturing delays, additional costs, or other difficulties. In addition, if we need to rapidly increase our business and manufacturing capacity to meet increases in demand or expedited shipment schedules, this may exacerbate any interruptions in our manufacturing operations and supply chain and the associated effect on our working capital.
If any of our customers cancel or fail to accept a large system order, our financial position and results of operations could be materially and adversely affected.
Our backlog, consists of orders for customized systems including our chemical vapor deposition equipment and annealing and diffusion furnaces which are built to client specifications. We also have a significant concentration of revenue with customers exceeding 10% of revenues. Two customers represented 30.5% in total and two customers represented 39.3%, respectively, of our annual revenues in fiscal years 2020 and 2019. These customized systems can have prices that range from $100,000 to several million dollars, depending on the configuration, specific options included and any special requirements of the customer. Because our orders are subject to cancellation or delay by the customer, our backlog at any particular point in time is not necessarily representative of actual sales for succeeding periods, nor does our backlog provide any assurance of achievement of revenues or that we will realize a profit from completing these orders. Our financial position and results of operations could be materially and adversely affected should any large system order be cancelled prior to shipment, or not be accepted by the customer due to alleged non-conformity with product specifications or otherwise. Likewise, a significant change in the liquidity or financial position of any of our customers that purchase large systems, could have a material impact on the collectability of our accounts receivable and our future operating results. Our backlog does not provide any assurance that we will realize a profit from those orders, or indicate in which period revenue will be recognized.
We may not be able to hire or retain the number of qualified personnel, particularly engineering personnel, required for our business, which would harm the development and sales of our products and limit our ability to grow.
Competition in our industry for senior management, technical, sales, marketing and other key personnel is intense. If we are unable to retain our existing personnel, or attract and train additional qualified personnel, our growth may be limited due to a lack of capacity to develop and market our products.
In particular, we have, from time to time, experienced difficulty in hiring and retaining skilled engineers with appropriate qualifications to support our growth strategy. Our success depends on our ability to identify, hire, train and retain qualified engineering personnel with experience in equipment design. Specifically, we need to continue to attract and retain mechanical, electrical, software and field service engineers to work with our direct sales force to technically qualify and perform on new sales opportunities and orders, and to demonstrate our products.
Our financial position and results of operations may be materially harmed if we are unable to recoup our investment in research and development.
The rapid change in technology in our industry requires that we continue to make substantial investments in research and development and selective acquisitions of technologies and products, in order to enhance the performance and functionality of our product line, to keep pace with competitive products and to satisfy customer demands for improved performance, features and functionality. These efforts include those related to the development of technology for the commercialization of carbon nanotubes. There can be no assurance that revenue from future products or enhancements will be sufficient to recover the development costs associated with such products, enhancements or acquisitions, or that we will be able to secure the financial resources necessary to fund future research and development or acquisitions. Research and development costs are typically incurred before we confirm the technical feasibility and commercial viability of a product, and not all development activities result in commercially viable products. In addition, we cannot ensure that products or enhancements will receive market acceptance, or that we will be able to sell these products at prices that are favorable to us. Our business could be seriously harmed if we are unable to sell our products at favorable prices, or if our products are not accepted by the markets in which we operate.
Our reputation and operating performance may be negatively affected if our products are not timely delivered.
We provide complex products that often require substantial lead-time for design, ordering parts and materials, and for assembly and installation. The time required to design, order parts and materials and to manufacture, assemble and install our products, may in turn lead to delays or shortages in the availability of some products. If a product is delayed or is the subject of shortage because of problems with our ability to design, manufacture or assemble the product on a timely basis, or if a product or software otherwise fails to meet performance criteria, we may lose revenue opportunities entirely, or experience delays in revenue recognition associated with a product or service. In addition, we may incur higher operating expenses during the period required to correct the problem.
Our business might be adversely affected by our dependence on foreign business.
During the year ended December 31, 2020, approximately $2.8 million or 16.8% of our revenues were generated by sales to customers outside the U.S. compared to approximately $4.2 million or 21.3% for the year ended December 31, 2019.
Because a portion of our revenues are derived from international customers, our operating results could be negatively affected by a decline in the economies of any of the countries or regions in which we do business. Each region can exhibit unique characteristics, which can cause capital equipment investment patterns to vary significantly from period to period. Periodic local or international economic downturns, trade balance issues and political instability, as well as fluctuations in interest and currency exchange rates, could negatively affect our business and results of operations.
All of our sales to date have been primarily priced in U.S. dollars. While our business has not been materially affected in the past by currency fluctuations, there is a risk that it may be materially adversely affected in the future. Such risks include possible losses due to both currency exchange rate fluctuations and from possible social and political instability.
If our critical suppliers fail to deliver sufficient quantities of quality materials and components in a timely and cost-effective manner, it could negatively affect our business.
We do not manufacture many components used in the production of our products, and consequently, we use numerous unrelated suppliers of materials and components. We generally do not have guaranteed supply arrangements with our suppliers. Because of the variability and uniqueness of our customer’s orders, we try to avoid maintaining an extensive inventory of materials and components for manufacturing. While we are not dependent on any principal or major supplier for most of our material and component needs, switching over to an alternative supplier may take significant amounts of time and added expense, which could result in a disruption of our operations and adversely affect our business.
It is not always practical or even possible to ensure that component parts are available from multiple suppliers; accordingly, we procure some key parts from a single supplier or a limited group of suppliers. At certain times, increases in demand for capital equipment can result in longer lead-times for many important system components, which may cause delays in meeting shipments to our customers. The delay in the shipment of even a few systems could cause significant variations in our quarterly revenue, operating results and the market value of our common stock.
We cannot assure you that our financial position and results of operations will not be materially and adversely affected if, in the future, we do not receive in a timely and cost-effective manner a sufficient quantity of quality component parts and materials to meet our production requirements.
The price of our common shares is volatile and could decline significantly.
The stock market in general and the market for technology stocks in particular has experienced volatility. If those industry-based market fluctuations continue, the trading price of our common shares could decline significantly independent of the overall market, and shareholders could lose all or a substantial part of their investment. The market price of our common shares could fluctuate significantly in response to several factors, including, among others:
|
● |
difficult macroeconomic conditions, unfavorable geopolitical events, and general stock market uncertainties, such as those occasioned by a global liquidity crisis and a failure of large financial institutions; |
|
● |
receipt of large orders or cancellations of orders for our products; |
|
● |
issues associated with the performance and reliability of our products; |
|
● |
actual or anticipated variations in our results of operations; |
|
● |
announcements of financial developments or technological innovations; |
|
● |
changes in recommendations and/or financial estimates by investment research analysis; |
|
● |
strategic transactions, such as acquisitions, divestitures, or spin-offs; and |
|
● |
the occurrence of major catastrophic events, including the effects of the spread of COVID-19 |
|
● |
trading volume is low |
Significant price and value fluctuations have occurred with respect to our publicly traded securities and technology companies generally. The price of our common shares is likely to be volatile in the future. In the past, securities class action litigation often has been brought against a company following periods of volatility in the market price of its securities. If similar litigation were pursued against us, it could result in substantial costs and a diversion of management’s attention and resources, which could materially and adversely affect our financial condition, results of operations, and liquidity.
We face the risk of product liability claims.
The manufacture and sale of our products, which in operation sometimes involve the use of toxic materials and extreme temperatures, and could result in product liability claims. For example, our rapid thermal processing systems used to heat semiconductor materials to temperatures in excess of 1000º Celsius have certain inherent risks. A failure of our product(s) at a customer site could also result in losses due to interruption of the business operations of our customer. While we regularly evaluate the nature and limits of our insurance coverages, there can be no assurance that our existing policies of insurance will be adequate to protect us from all liabilities that we might incur in connection with the manufacture and sale of our products in the event of a successful product liability claim or series of successful claims against us.
We are subject to environmental regulations, and our inability or failure to comply with these regulations could adversely affect our business.
We are subject to environmental regulations in connection with our business operations, including regulations related to the development and manufacture of our products and our customers’ use of our products. Our failure or inability to comply with existing or future environmental regulations could result in significant remediation liabilities, the imposition of fines or the suspension or termination of development, manufacturing or use of certain of our products, or affect the operation of our facilities, use or value of our real property, each of which could damage our financial position and results of operations.
If we are subject to cyber-attacks we could incur substantial costs and, if such attacks are successful, we could incur significant liabilities, reputational harm, and disruption to our operations.
We manage, store and transmit proprietary information and sensitive data relating to our operations. We may be subject to breaches of the information technology systems we use for these purposes. Experienced computer programmers and hackers may be able to penetrate our network security and misappropriate and/or compromise our confidential information (and or third-party confidential information), create system disruptions, or cause shutdowns. Computer programmers and hackers also may be able to develop and deploy viruses, worms, and other malicious software programs that attack our systems or our products, or that otherwise exploit any security vulnerabilities.
The costs to address the foregoing security problems and security vulnerabilities before or after a cyber-incident could be significant. Our remediation efforts may not be successful and could result in interruptions, delays, or cessation of service, and loss of existing or potential customers, impeding our sales, manufacturing, distribution, or other critical functions. In addition, breaches of our security measures and the unapproved dissemination of proprietary information or sensitive data about us, our customer, or other third parties, could expose us, our customers, or other third parties to a risk of loss or misuse of this information, result in litigation and potential liability for us, damage our reputation, or otherwise harm our business.
Regulations related to conflict minerals will force us to incur additional expenses, may make our supply chains more complex, and may result in damage to our relationships with customers.
Under the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010, or the Dodd-Frank Act, the SEC adopted requirements for companies that manufacture products that contain certain minerals and metals known as "conflict minerals”. These rules require public companies to perform diligence and to report annually to the SEC whether such minerals originate from the Democratic Republic of Congo and adjoining countries. The implementation of these requirements could adversely affect the sourcing, availability, and pricing of minerals we use in the manufacture of our products. In addition, we have incurred and will continue to incur additional costs to comply with the disclosure requirements, including costs related to determining the source of any of the relevant minerals used in our products. Given the complexity of our supply chain, we may not be able to ascertain the origins of these minerals used in our products through the due diligence procedures that we implement, which may harm our reputation. We may also face difficulties in satisfying customers who may require that our products be certified as conflict mineral free, which could harm our relationships with these customers and lead to a loss of revenue. These requirements could limit the pool of suppliers that can provide conflict-free minerals, and we may be unable to obtain conflict-free minerals at competitive prices, which could increase our costs and adversely affect our manufacturing operations and our profitability.
Failure to comply with the United States Foreign Corrupt Practices Act could subject us to penalties and other adverse consequences.
We are subject to the United States Foreign Corrupt Practices Act, which generally prohibits United States companies from engaging in bribery or other prohibited payments to foreign officials for the purpose of obtaining or retaining business. We have agreements with third parties and make sales in countries known to experience corruption, extortion, bribery, pay-offs, theft and other fraudulent practices. We can make no assurance, however, that our employees or other agents will not engage in such conduct for which we might be held responsible. If our employees or other agents are found to have engaged in such practices, we could suffer severe penalties and other consequences that may have a material adverse effect on our business, financial condition and results of operations.
Item 1B. Unresolved Staff Comments
None.
Item 2. Description of Property.
|
Owned Locations |
Size (sf) |
Segment |
Mortgage/Loan |
Principal use |
||
|
Central Islip, NY |
130,000 |
CVD Equipment |
Yes |
Corporate: R&D; Manufacturing |
||
|
Central Islip, NY |
179,000 |
CVD Materials |
Yes |
Manufacturing and R&D |
||
|
Saugerties, NY |
22,000 |
SDC |
No |
Admin; Manufacturing |
|
Leased Locations |
Size (sf) |
Segment |
Lease term |
Principal use |
||
|
Nordborgvej, |
||||||
|
Denmark |
7,793 |
CVD Materials |
1 year |
Process coatings, |
||
|
Admin |
Item 3. Legal Proceedings.
Not applicable.
Item 4. Mine Safety Disclosures.
Not applicable.
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Our common stock is listed on the NASDAQ Capital Market under the symbol "CVV.” The following table sets forth, for the periods indicated, the high and low prices of our common stock on the NASDAQ Capital Market.
|
High |
Low |
|||||||
|
Year Ended December 31, 2020: |
||||||||
|
1st Quarter |
$ | 5.59 | $ | 1.95 | ||||
|
2nd Quarter |
4.10 | 2.20 | ||||||
|
3rd Quarter |
4.70 | 2.64 | ||||||
|
4th Quarter |
7.47 | 2.85 | ||||||
|
High |
Low |
|||||||
|
Year Ended December 31, 2019: |
||||||||
|
1st Quarter |
$ | 4.75 | $ | 3.38 | ||||
|
2nd Quarter |
3.98 | 3.44 | ||||||
|
3rd Quarter |
3.80 | 3.25 | ||||||
|
4th Quarter |
4.00 | 3.10 | ||||||
As of March 24, 2021, there were approximately 91 holders of record and approximately 1,200 beneficial owners of our common stock, and the closing sales price of our common stock as reported on the NASDAQ Capital Market was $4.33.
Dividend Policy
We have never paid dividends on our common stock and we do not anticipate paying dividends on common stock at the present time. We currently intend to retain earnings, if any, for use in our business. There can be no assurance that we will ever pay dividends on our common stock. Our dividend policy with respect to our common stock is within the discretion of the Board of Directors and its policy with respect to dividends in the future will depend on numerous factors, including earnings, financial requirements and general business conditions.
Equity Compensation Plan Information Table
The following table provides information about shares of our common stock that may be issued upon the exercise of options under all of our existing compensation plans as of December 31, 2020.
|
Number of securities to be issued upon exercise of outstanding options, warrants and rights(1) |
Weighted-average exercise price of outstanding options, warrants and rights(2) |
Number of securities remaining available for future issuance |
||||||||||
|
Plan Category |
||||||||||||
|
Equity compensation plans approved by security holders |
417,000 | $ | 11.26 | 513,188 | ||||||||
|
Equity compensation plans not approved by security holders |
-- | N/A | -- | |||||||||
|
Total |
417,000 | $ | 11.26 | 513,188 | ||||||||
(1) Reflects aggregate options and restricted stock awards outstanding under our 2001 Stock Option Plan, 2007 Share Incentive Plan and 2016 Equity Incentive Plan.
(2) Calculation is exclusive of the value of any unvested restricted stock awards.
Recent Sales of Unregistered Securities
None.
Issuer Purchases of Equity Securities
None.
Item 6. Selected Financial Data.
None.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
Except for historical information contained herein, this "Management’s Discussion and Analysis of Financial Condition and Results of Operations” contains forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995, as amended. These statements involve known and unknown risks, uncertainties and other factors which may cause the actual results, performance, or achievements of the Company to be materially different from any future results, performance, or achievements expressed or implied by such forward-looking statements. These forward-looking statements were based on various factors and were derived utilizing numerous important assumptions and other important factors that could cause actual results to differ materially from those in the forward-looking statements. Important assumptions and other factors that could cause actual results to differ materially from those in the forward-looking statements, include but are not limited to: competition in the Company’s existing and potential future product lines of business; the Company’s ability to obtain financing on acceptable terms if and when needed; uncertainty as to the Company’s future profitability, uncertainty as to the future profitability of acquired businesses or product lines, uncertainty as to any future expansion of the Company and the effect of the novel coronavirus (COVID-19) on our business and operations, and those of our customers, suppliers and other third parties . Other factors and assumptions not identified above were also involved in the derivation of these forward-looking statements and the failure of such assumptions to be realized as well as other factors may also cause actual results to differ materially from those projected. The Company assumes no obligation to update these forward-looking statements to reflect actual results, changes in assumptions or changes in other factors affecting such forward-looking statements. Past results are no guaranty of future performance. You should not place undue reliance on any forward-looking statements, which speak only as of the dates they are made. When used with this Report, the words "believes”, "anticipates”, "expects”, "estimates”, "plans”, "intends”, "will” and similar expressions are intended to identify forward-looking statements.
Executive Level Summary
We develop, design, manufacture and service a broad range of chemical vapor deposition, gas control and other state-of-the-art process equipment and solutions used in research & development and production of advanced materials and coatings. Our served markets extend from research to industrial applications. This equipment is used by our customers to research, design, and manufacture these materials or coatings for aerospace engine and structural components, medical devices such as implants, advanced semiconductor devices, solar cells, smart glass, carbon nanotubes, nanowires, LEDs, MEMS and other applications. Through CVD Materials and our Application Laboratory, we develop new material systems, provide material coating services, process development support and process startup assistance with the focus on enabling tomorrow’s technologiesTM.
Based on more than 39 years of experience, we use our capabilities in process development, engineering and manufacturing to transform new applications into leading-edge manufacturing solutions. This enables university, research and industrial scientists at the cutting edge of technology to develop next generation aerospace, medical, nano, LEDs, semiconductors and other electronic components. We develop, manufacture and provide equipment for research and production based on our proprietary designs. We have built a significant library of design expertise, know-how and innovative solutions to assist our customers in developing these intricate processes and to accelerate their commercialization. This library of equipment design solutions, along with our manufacturing and systems integration facilities, allows us to provide superior design, process and manufacturing solutions to our customers.
Our strategy is to target opportunities in the research, development and production equipment market, with a focus on high growth applications such as aerospace, carbon nanotubes, nanowires, medical, graphene, MEMS and LEDs. To expand our penetration into these growth markets, we have developed a line of proprietary standard products and custom systems. Historically, we manufactured products for research and development on an applications specific basis to meet an individual customer’s specific research requirements. Our proprietary systems leverage the technological expertise that we have developed through designing these custom systems onto a standardized basic core. This core is easily adapted through a broad array of available options to meet the diverse product and budgetary requirements of the research community. By manufacturing the basic core of these systems in higher volumes, we are able to reduce both the cost and delivery time for our systems. These systems, which we market and sell under the EasyTube® and CVD product lines, are sold to researchers at universities, research laboratories, and startup companies in the United States and throughout the world.
Sales of our proprietary standard systems, custom systems and process solutions have been driven by our installed customer base, which includes Fortune 500 companies. The strong performance and success of our products has historically driven repeat orders from existing customers as well as business from new customers. Furthermore, with our proprietary solutions and expanded focus on "accelerating the commercialization of tomorrow’s technologies”TM we have been developing a new customer base in addition to growing with our existing customers. We have generally gained new customers through our reputation in the markets and industries, limited print advertising and trade show attendance (which has been negatively impacted by COVID-19).
The core competencies we have developed in equipment and software design, as well as in systems manufacturing and process solutions, are used to engineer our finished products and to accelerate the commercialization path of our customer base. Our proprietary-real-time, software allows for rapid configuration, and provides our customers with powerful tools to understand, optimize and repeatedly control their processes. These factors significantly reduce cost, improve quality and reduce the time it takes from customer order to shipment of our products. Our Application Laboratory allows customers the option to bring up their process tools in our Application Laboratory and to work together with our scientists and engineers to optimize process performance.
Current Developments
Historically, we have derived substantially all of our revenues through our custom equipment business and our Stainless Design Concepts ("SDC”) gas management and chemical delivery control systems segment. The marketing, sale and manufacture of our products, requires a lengthy sales cycle ranging from several months to over more than one year before we can complete production and delivery. Also, demand for our equipment and related consumable products and services may be volatile as a result of sudden changes in market conditions, competition and other factors. This can and has resulted in substantial volatility in our revenue stream.
In order to address this sales volatility, we have attempted to diversify and expand our business into providing material products and services. This strategy included the development of our capabilities to provide materials coatings and surface treatments for targeted customer / market requirements (the "Material Business”). With this objective in mind, we acquired Tantaline in December 2016 and MesoScribe in October 2017. In order to facilitate these new lines of businesses, we also purchased a building to house both operating subsidiaries for $13,850,000. This 180,000 square foot building (the "555 Building”) was to house the Material Business in the United States and provide adequate space for the anticipated growth of these businesses. In addition, we also maintain a 130,000 square foot building (the "355 Building”), which houses the equipment products portion of our business as well as our corporate headquarters.
We have invested approximately $1.6 million, $2.7 million and $2.5 million during 2020, 2019 and 2018, respectively, in building improvements, machinery, and other expenses related primarily to the Materials Business.
The projected growth of the Materials Business has not met expectations. Although we have made substantial investments in facilities, equipment and acquisitions in furtherance of our strategy, the foregoing has proven to be a significant drain on our finances and our liquidity. Since 2018 revenues for the Materials Business have been $1,700,000 in 2018, $1,600,000 in 2019 and $2,300,000 in 2020, with operating losses, exclusive of a $3.6 million impairment charge, recorded in all years for a total loss of $2.5 million. These cumulative results are due to operating losses from the Tantaline operations offset by operating profits of $.5 million from the MesoScribe operations.
Furthermore, our overall revenues have declined from $41.1 million in 2017 to $16.9 million in 2020. Cumulative operating losses, exclusive of a $3.6 million impairment charge, for the last three years (2018-2020) totaled ($14.5 million), which are comprised of 2018 ($5.3 million), 2019 ($5.0 million) and 2020 ($4.2 million). As a result of these continuing losses, and the investments in the Materials Business, our cash balances have declined from $21.7 million at December 31, 2016 to $7.7 million as of December 31, 2020, and liquidity has been strained. Contributing to and compounding this decline, is the negative effect the COVID-19 crisis has had in 2020 on the aerospace industry, which resulted from reduced travel and reduction of industry gas turbine engine sales. Aerospace sales in recent years have represented as much as 60% of our total revenue.
Our mortgage debt on the 355 Building and 555 Building, in the amount of $2.1 million and $9.3 million respectively, at December 31 2020, matures in March and December 2022, respectively.
In January 2021, our Board of Directors concluded that we needed a change in direction and new leadership to evaluate our business strategy and operations, and take timely actions to halt and reverse the declines of the past few years. As such, they appointed Emmanuel Lakios as President and Chief Executive Officer (previously our Vice-President- Sales and Marketing). We began an intensive analysis of our entire business and operations including the Materials Business. Based upon that analysis we believe our primary focus should be on the core equipment business and that the Materials Business strategy should be revised, with some of its current elements potentially minimized or ceased. Based upon this analysis, we are forecasting continued losses and negative cash flow for our Tantaline product line and as a consequence, we have implemented plans to eliminate further investment in our Tantaline product line, which will result in the avoidance of approximately $1.5-$2.0 million in additional costs. In addition, we have recorded an impairment charge of $3.6 million during the fourth quarter and year ended December 31, 2020. Based upon certain decisions and actions currently being reviewed, there may be additional costs to be incurred, inclusive of employee related and lease termination costs estimated at approximately $400,000.
In order to increase our liquidity and to provide necessary working capital to support our on-going business and operations, we have decided to sell the 555 Building in February 2021. We have determined the 555 Building is not needed for present and future business operations. We have concluded that any remaining elements of the Materials Business can be consolidated into the 355 Building, which we believe can accommodate any needs for our growth for the foreseeable future.
On March 29, 2021, the Company entered into an agreement with Steel K, LLC for the sale of its 555 Building. The purchase price is $24,360,000, and the closing of the sale is subject to the satisfaction or waiver of certain conditions to closing or contingencies. A portion of the sale proceeds would be used to satisfy the existing mortgage debt on the 555 Building in the approximate amount of $9.3 million at December 31, 2020, and for various costs related to the sale closing in an amount to be determined. Any excess proceeds will be used for general working capital purposes.
Statement of Operations
|
2020 |
2019 |
|||||||
|
Revenue |
$ | 16,920,219 | $ | 19,646,652 | ||||
|
Cost of revenue |
14,037,813 | 16,850,077 | ||||||
|
Gross profit |
2,882,406 | 2,796,575 | ||||||
|
Operating expenses |
||||||||
|
Research and development |
372,648 | 597,456 | ||||||
|
Selling and shipping |
580,468 | 898,338 | ||||||
|
Impairment charge |
3,599,322 | - | ||||||
|
General and administrative |
6,153,925 | 6,285,496 | ||||||
|
Total operating expenses |
10,706,363 | 7,781,290 | ||||||
|
Operating loss |
(7,823,957 | ) | (4,984,715 | ) | ||||
|
Other income (expense): |
||||||||
|
Interest income |
62,667 | 142,579 | ||||||
|
Interest expense |
(444,337 | ) | (482,844 | ) | ||||
|
Other income |
603,320 | 411,230 | ||||||
|
Total other income, net |
221,650 | 70,965 | ||||||
|
Loss before income tax |
(7,602,307 | ) | (4,913,750 | ) | ||||
|
Income tax (benefit) expense |
(1,527,355 | ) | 1,413,908 | |||||
|
Net loss |
$ | (6,074,952 | ) | $ | (6,327,658 | ) | ||
Revenue
|
2020 |
2019 |
Change |
%Change |
|||||||||||||
|
CVD Equipment |
$ | 10,385,107 | $ | 14,065,112 | $ | (3,680,005 | ) | (26.2 | %) | |||||||
|
SDC |
4,207,182 | 3,941,946 | 265,236 | 6.7 | % | |||||||||||
|
CVD Materials |
2,327,930 | 1,639,594 | 688,336 | 42.0 | % | |||||||||||
|
Total |
$ | 16,920,219 | $ | 19,646,652 | $ | (2,726,433 | ) | (13.9 | %) | |||||||
Our revenue for the year ended December 31, 2020 was $16.9 million compared to $19.6 million for the year ended December 31, 2019, resulting in a decrease of 13.9%. This was primarily attributable to decreased revenue of $3.7 million from our CVD Equipment segment related to spare parts and equipment sales, offset, in part by, an increase of $.3 million in our SDC segment and an increase of $.7 million in our CVD Materials segment. Contributing to and compounding this decline, is the negative effect the COVID-19 crisis has had in 2020 on the aerospace industry, which resulted from reduced travel and reduction of industry gas turbine engine sales. Aerospace sales have represented as much as 60% of our total revenue.
The revenue contributed for the year ended December 31, 2020, by the CVD Equipment segment, was $10.4 million, or 61.4% of our overall revenue. This represented a decrease of 26.2% or $3.7 million, as compared to $14.1 million in the prior year, which totaled 71.6% of our overall revenue. This revenue decrease is the result of decreases of $.9 million and $2.8 million, from spare parts and equipment sales, respectively. Contributing to and compounding this decline, is the negative effect the COVID-19 crisis has had in 2020 on the aerospace industry, which resulted from reduced travel and reduction of industry gas turbine engine sales. Aerospace sales have represented as much as 60% of our total revenue.
Annual revenue for our SDC segment increased to $4.2 million in 2020 as compared to $3.9 million in 2019, an increase $.3 million or 6.7%. The SDC segment represented 24.9% and 20.0% of our total revenue during the years ended December 31, 2020 and December 31, 2019, respectively.
Revenues for our CVD Materials segment were $2.3 million in the year ended December 31, 2020 as compared to $1.6 million for 2019. The increase of $.7 million was due to increased sales of Tantaline products and coatings of $.6 million, half of this increase related to a distribution sale at significantly reduced margins, and increased sales from MesoScribe of $.1 million.
Gross Profit
Gross profit for the year ended December 31, 2020 amounted to $2.9 million, with a gross profit margin of 17.0 %, compared to a gross profit of $2.8 million and a gross profit margin of 14.2% for the year ended December 31, 2019. The increase in our gross profit and gross profit margin was the result of improvements in our operating efficiencies with certain repeat orders, as well as lowered costs mostly due to the effects of employees furloughed during the period as a result of Coronavirus mandates imposed, and improved mix of product revenues resulting in our gross profit margin percentage improvement.
Research and Development, Selling and General and Administrative Expenses
Research and Development:
Due to the technical development required on our custom orders, our research and development team and their expenses are charged to costs of goods sold when they are working directly on a customer project. When they are not working on a customer project, they work in our Application Laboratory and their costs are charged to research and development. For the years ended December 31, 2020 and 2019, we incurred $373,000 and $598,000 respectively of internal research and development costs. This decrease was primarily due to the effects of employee furloughs during the year ended December 31, 2020, as a result of COVID-19 mandates imposed.
Selling:
Selling expenses were $.6 million or 3.4% of the revenue for the year ended December 31, 2020 as compared to $.9 million or 4.6% for the year ended December 31, 2019. The decrease was primarily the result of reduced employee related costs, including the effects of employee furloughs during the year ended December 31, 2020, as a result of COVID-19 mandates imposed, and lower trade show expenses.
Impairment Charge:
Based upon continued operating losses and negative cash flow for our Tantaline product line and our updated forecasting, the expected future cash flows of the Tantaline business is negative and thus we have recorded an impairment charge of $3.6 million in the fourth quarter and year ended December 31, 2020. We had no recorded impairment charges in the consolidated statement of operations during the year ended December 31, 2019.
General and Administrative:
General and administrative expenses for the year ended December 31, 2020 were $6.2 million or 36.4% of revenue compared to $6.3 million or 32.0% during the year ended December 31, 2019, a decrease of $.1 million. While stock compensation costs decreased by $317,000 due to fewer equity grants, and outside systems and finance consulting costs decreased by $67,000, due to the completion of our system migration and completion of finance consulting costs in 2019, these decreases were offset primarily by depreciation of our 555 facility in the amount of $334,000 and a $142,000 bad debt provision related primarily to one customer.
Operating Loss
Improved gross profit margins and reduced expenses, which were more than offset by the $3.6 million impairment charge in the year ended December 31, 2020, resulted in an operating loss of $7.8 million for the year ended December 31, 2020 as compared to operating loss of $5.0 million for the year ended December 31, 2019.
Other (Expenses)/Income
Other income was $221,650 and $70,965 for the years ended December 31, 2020 and 2019, respectively. Net other income was $603,320 and $411,230 for the year ended December 31, 2020 and 2019, respectively, from subleasing a portion of our CVD Materials facility. The increase of $192,090 was the result of approximately seven months of rental in 2019 (commencing June 2019) as compared to a full year in 2020. Interest income of $62,667 for the year ended December 31, 2020, included interest income of $28,000 related to the income tax refund received during the year. As a result of lower interest rates, interest income decreased $107,912, to $34,667 for the year ended December 31, 2020 as compared to $142,579 in 2019. In addition, interest expense decreased $38,507 to $444,337 in the year ended December 31, 2020, as compared to $482,844 in 2019.
Income Taxes
For the year ended December 31, 2020, we recorded an income tax benefit of $1,527,000 as compared to an income tax expense of $1,414,000 in the year ended December 31, 2019. The income tax benefit recorded during the year ended December 31, 2020 was the result of a change in the tax laws pursuant to the CARES Act. As a result of the enactment of the CARES Act, net operating losses ("NOL’s”) generated in 2018-2020 can now be carried back for five years and resulted in the Company recognizing approximately a $1.5 million income tax benefit, of which $.7 million was a receivable at December 31, 2020. As of December 31, 2020 and December 31, 2019, we have provided a full valuation allowance against all of the net deferred tax assets. This was based on management’s assessment, including three years of cumulative operating losses, that it is more likely than not that the net deferred tax assets may not be realized in the future. For the year ended December 31, 2019, we have provided a full valuation allowance against all of the net deferred tax assets in the amount of $2,497,414. We continue to evaluate for potential utilization of our deferred tax asset, which has been fully reserved for, on a quarterly basis, reviewing our economic models, including projections and timing of orders, cost containment measures and other factors. For the year ended December 31, 2020 and 2019 our tax rate was primarily affected by permanent differences resulting in an effective tax rate of 20.0% and 28.7%.
Net Income Loss
As a result of the foregoing factors, for the year ended December 31, 2020, we had a net loss of $6.1 million or $.91 per diluted share compared to a net loss of $6.3 million or $.96 per diluted share for the year ended December 31, 2019.
Inflation
Inflation has not materially impacted our operations.
Liquidity and Capital Resources
As of December 31, 2020, we had aggregate working capital of $8.1 million compared to aggregate working capital of $8.8 million at December 31, 2019. Our cash and cash equivalents at December 31, 2020 and 2019 were $7.7 million and $8.7 million, respectively.
Net cash used in operating activities was ($1.1 million). This is the result of the net loss, adjusted for non-cash items, of $.7 million. In addition, contract liabilities decreased $1.5 million, accrued expenses decreased $.5 million related to the payment of vacation and other accrued expenses and an increase in taxes receivable of $.7 million as a result of the March 27, 2020 CARES Act enactment allowing the carryback of NOL’s five years resulting in a receivable of $1.5 million, of which $.8 million was collected in the year ended December 31, 2020. These amounts were reduced by a decrease in accounts receivable of $1.4 million due to timing of collections, decreased inventory of $.6 million and increased accounts payable of $.3 million. Our cash and cash equivalents at our year end December 31, 2020 was $7.7 million and was favorably impacted by the receipt of the PPP loan proceeds of $2.4 million and the refund of income taxes of $.8 million for a total of $3.2 million.
Long term debt increased by $1.7 million, the result of a new loan from the Paycheck Protection Program of $2.4 million and a ($.7 million) decrease from principal payments on the mortgages related to our two facilities in Central Islip, NY, including our investment in the CVD Materials building purchased on November 30, 2017. We have invested in activities primarily related to preparing CVD Materials for operations in the United States. Our total capital invested in the year ended December 31, 2020 was $1.6 million, primarily related to building improvements and machinery, and for the year ended December 31, 2020 we received rental income of approximately $603,000.
We have a loan agreement with HSBC USA, N.A. (the "HSBC”) which is secured by a mortgage on our Central Islip headquarters at 355 South Technology Drive. The loan is payable in 120 consecutive equal monthly installments of $25,000 in principal plus interest and a final balloon payment upon maturity in March 2022. The balances as of December 31, 2020 and December 31, 2019 were approximately $2.1 million and $2.4 million, respectively. Interest accrues on the Loan, at our option, at the variable rate of LIBOR plus 1.75% or Prime less 0.5% (1.89% and 3.49% at December 31, 2020 and 2019, respectively).
On November 30, 2017, we purchased the premises located at 555 North Research Place, Central Islip, NY which is intended to house the CVD Materials segment. The purchase price of the land and the building was $13,850,000 exclusive of closing costs.
As part of the acquisition, our newly formed wholly-owned subsidiary, 555 N Research Corporation (the” Assignee”) and the Islip IDA, entered into a Fee and Leasehold Mortgage and Security Agreement (the ”Loan”) with HSBC in the amount of $10,387,500, which was used to finance a portion of the purchase price to acquire the premises located at 555 North Research Place, Central Islip, New York (the ”Premises”). The Loan was evidenced by the certain Note, dated November 30, 2017 (the ”Note”), by and between Assignee and the Bank, and secured by a certain Fee and Leasehold Mortgage and Security Agreement, dated November 30, 2017 (the "Mortgage”), as well as a collateral Assignment of Leases and Rents ("Assignment of Leases”).
The Note is payable in 60 consecutive equal monthly installments of $62,481, including interest.
The balances as of December 31, 2020 and 2019 were approximately $9.3 million and $9.7 million, respectively. The Note bears interest for each Interest Period (as defined in the Note), at the fixed rate of 3.9148%. The maturity date for the Note is December 1, 2022. As a condition of the Bank making the Loan, we were required to guaranty Assignee’s obligations under the Loan.
On August 5, 2019, we entered into a Mortgage Modification Agreement which replaced the former covenant with a Minimum Liquid Assets ("MLC”) covenant, and on October 22, 2020, we entered into a Second Mortgage Modification Agreement modifying certain MLC balances. We were in compliance with our financial covenant under the mortgage at December 31, 2020.
The COVID-19 outbreak has resulted in extended shutdowns of certain businesses in United States and around the world.. We have been actively monitoring the COVID-19 outbreak and its impact globally. Our primary focus to this point has been to ensure the health and safety of our employees. To that end, we have adopted social distancing where appropriate, implemented travel restrictions, and we have taken actions to ensure that locations and facilities are cleaned and sanitized regularly. These are novel and challenging times and the magnitude of this crisis is requiring us to consider all options to promote the safety of employees, including, where appropriate, or where required to comply with foreign, national, state or local governmental authority recommendations, guidelines, and/or mandates, the temporary reduction or suspension of work at certain of the Company’s locations and production facilities to protect employees and curb the spread of the coronavirus. All of these actions have adversely impacted our operating results. In particular, our aerospace sector, for which we rely on a significant part of our business, has been faced with significant reductions to its business due to lack of air travel. Due to the timing of the COVID-19 outbreak, our new order levels during the year ended December 31, 2020, and into 2021 have seen substantial reductions which have materially and adversely affected revenues commencing in our second quarter of 2020, and is anticipated to continue into 2021. While the financial results for our first quarter of 2020 reflected the initial impact of COVID-19, and the year ended December 31, 2020 reflected a substantial adverse effect, we are unable to predict the extent of the impact the pandemic will have on our financial position and operating results for the 2021 due to numerous uncertainties, but the impact could be material and adverse during any future period affected either directly or indirectly by this pandemic. The longer-term impacts from the outbreak are highly uncertain and cannot be predicted. Our return to profitability is dependent upon, among other things, the receipt of new equipment orders, the lessening of the ongoing effects of COVID-19 on our business and the Aerospace market, improvement in the operations of the materials business, the consolidation of our Central Islip facilities and sale of the 555 Building, as well as managing planned capital expenditures and operating expenses.
At December 31, 2019 we had reduced our employee headcount by 13% to 172 as compared to December 31, 2018. Since March 16, 2020, as a result of Coronavirus mandates imposed, we have furloughed a substantial portion of our work force reducing to levels deemed to support essential services, and continue to assess this on a weekly basis. During these unprecedented times we are continuing to evaluate our staffing levels to support the continued operations, including the level of current and expected orders. As of December 31, 2020, our active employee headcount has been reduced to approximately 130, a 24% reduction as compared to December 31, 2019.
On April 21, 2020, the Company entered into a loan agreement (the "Loan Agreement”) with HSBC Bank USA, National Association pursuant to which the Company was granted a loan in the principal amount of $2,415,970, pursuant to the Paycheck Protection Program (the "PPP”) under Division A, Title I of the CARES Act, which was enacted by the United States Congress on March 27, 2020.
The PPP loan, the obligation of which is represented by a note issued by the Company, matures on April 21, 2022 and bears interest at a rate of 1% per annum. The note may be prepaid by the Company at any time prior to maturity with no prepayment penalties. Under the terms of the PPP, all or a portion of the Loan may be forgiven, based upon payments made in the first twenty-four weeks following receipt of the proceeds, related to payroll costs, continue group health care benefits, utilities and mortgage interest on other debt obligations incurred before February 15, 2020. The Company is in process of filing its application for forgiveness and anticipates all or substantially all of the PPP loan to be forgiven.
As a result of the March 27, 2020 CARES Act enactment allowing the carryback of NOL’s five years, the Company recognized a $1.5 million tax benefit. The Company has collected $.8 million in the year ended December 31, 2020.
2021 Update
In January 2021, our Board of Directors concluded that we needed a change in direction and new leadership to evaluate our business strategy and operations, and take timely actions to halt and reverse the declines of the past few years. As such, they appointed Emmanuel Lakios as President and Chief Executive Officer (previously our Vice-President- Sales and Marketing). We began an intensive analysis of our entire business and operations including the Materials Business. Based upon that analysis we believe our primary focus should be on the core equipment business and that the Materials Business strategy should be revised, with some of its current elements potentially minimized or ceased. Based upon this analysis, we are forecasting continued losses and negative cash flow for our Tantaline product line and as a consequence, we have implemented plans to eliminate further investment in our Tantaline product line, which will result in the avoidance of approximately $1.5-$2.0 million in additional costs. In addition, we have recorded an impairment charge of $3.6 million in the fourth quarter and year ended December 31, 2020. Based upon certain decisions and actions currently being reviewed, there may be additional costs to be incurred, inclusive of employee related and lease termination costs estimated at approximately $400,000.
In order to increase our liquidity and to provide necessary working capital to support our on-going business and operations, we have decided to sell the 555 Building. We have determined the 555 Building is not needed for present and future business operations. We have concluded that any remaining elements of the Materials Business can be consolidated into the 355 Building, which we believe can accommodate any needs for our growth for the foreseeable future.
On March 29, 2021, the Company entered into an agreement with Steel K, LLC for the sale of its 555 Building. The purchase price is $24,360,000, and the closing of the sale is subject to the satisfaction or waiver of certain conditions to closing or contingencies. A portion of the sale proceeds would be used to satisfy the existing mortgage debt on the 555 Building in the approximate amount of $9.3 million at December 31, 2020, and for various costs related to the sale closing in an amount to be determined. Any excess proceeds will be used for general working capital purposes.
Due to the timing of the COVID-19 outbreak, our new orders during the year ended December 31, 2020, and into the beginning of 2021 have decreased substantially which have resulted in substantial reductions in revenues resulting in operating losses commencing in our second quarter of 2020. The ongoing impact that COVID-19 has had on our business has made the conditions to operate very challenging. In particular, the aerospace sector, for which we rely on a significant part of our business, has been faced with significant reductions to its business due to lack of air travel. While we continue to monitor and take action to reduce our expenses, we have secured a $2.4 million loan under PPP and have recognized a $1.5 million tax receivable from the NOL 5 year carryback. In addition, we have decided to sell the 555 Building. Based upon all of these factors, we believe that our cash and cash equivalent positions and cash flow from operations will be sufficient to meet our working capital and capital expenditure requirements for the next twelve months of the filing of this Form 10-K. Should the current environment continue longer or worsen, we will continue to assess our operations and take actions anticipated to maintain our operating cash to support the working capital needs, as well as compliance with our loan covenant.
Critical Accounting Policies
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. Our significant estimates include accounting for certain items such as revenues on long-term contracts recognized on the input method; valuation of inventories at the lower of cost or realizable value; allowance for doubtful accounts receivable; valuation of stock-based compensation; estimated lives and recoverable value of our long-lived assets and certain components of the deferred income tax provisions which are based on estimates of future taxable events.
Revenue Recognition
We design, manufacture and sell custom chemical vapor deposition equipment through contractual agreements. These system sales require us to deliver functioning equipment that is generally completed within three to eighteen months from commencement of order acceptance. We recognize revenue over time by using an input method based on costs incurred as it depicts our progress toward satisfaction of the performance obligation. Under this method, revenue arising from fixed price contracts is recognized as work is performed based on the ratio of costs incurred to date to the total estimated costs at completion of the performance obligations.
Incurred costs include all direct material and labor costs and those indirect costs related to contract performance, such as indirect labor, supplies, tools, repairs and depreciation costs. Contract material costs are included in incurred costs when the project materials have been purchased or moved to work in process as required by the project’s engineering design. Cost based input methods of revenue recognition require us to make estimates of costs to complete the projects. In making such estimates, significant judgment is required to evaluate assumptions related to the costs to complete the projects, including materials, labor and other system costs. If the estimated total costs on any contract are greater than the net contract revenues, we recognize the entire estimated loss in the period the loss becomes known and can be reasonably estimated.
We have been engaged in the production and delivery of goods on a continual basis under contractual arrangements for many years. Historically, we have demonstrated an ability to accurately estimate total revenues and total expenses relating to our long-term contracts. However, there exist many inherent risks and uncertainties in estimating revenues, expenses and progress toward completion, particularly on larger or longer-term contracts. If we do not estimate the total sales, related costs and progress toward completion on such contracts, the estimated gross margins may be significantly impacted or losses may need to be recognized in future periods. Any such resulting changes in margins or contract losses could be material to our results of operations and financial condition.
Stock-Based Compensation
We record stock-based compensation in accordance with the provisions set forth in the Financial Accounting Standard Board ("FASB”) Accounting Standards Codification ("ASC”) 718, "Stock Compensation”. ASC 718 requires companies to recognize the cost of employee services received in exchange for awards of equity instruments based upon the grant date fair value of those awards.
Long-Lived Assets and Intangibles
Long-lived assets consist primarily of property, plant and equipment. Intangibles consist of patents, copyrights, intellectual property, licensing agreements and certifications. Long-lived assets are reviewed for impairment whenever events or circumstances indicate their carrying value may not be recoverable. When such events or circumstances arise, an estimate of the future undiscounted cash flows produced by the asset, or the appropriate grouping of assets, is compared to the asset’s carrying value to determine if impairment exists pursuant to the requirements of ASC 360-10-35, "Impairment or Disposal of Long-Lived Assets.” If the asset is determined to be impaired, the impairment loss is measured on the excess of its carrying value over its fair value. Assets to be disposed of are reported at the lower of their carrying value or net realizable value. Based upon continued operating losses and our updated forecasting, the future value of the cash flows of the Tantaline product line is negative and thus we have recorded an impairment charge of $3.6 million in the fourth quarter and year ended December 31, 2020. We had no recorded impairment charges in the consolidated statement of operations during the year ended December 31, 2019.
Off-Balance Sheet Arrangements
None.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
Not applicable.
Item 8. Financial Statements and Supplementary Data.
The consolidated financial statements and supplementary data required by this item are included in this annual report beginning on page F-1.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Disclosure Controls and Procedures. We maintain a system of disclosure controls and procedures (as defined in Rule 13a-15(e) and 13d-15(e) under the Exchange Act of 1934, as amended, (the "Exchange Act”)). As required by Rule 13a-15(b) under the Exchange Act, management of the Company, under the direction of our Chief Executive Officer and Chief Financial Officer, reviewed and performed an evaluation of the effectiveness of design and operation of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Exchange Act) as of December 31, 2020.
Based on that review and evaluation, our Chief Executive Officer and Chief Financial Officer, along with others in our management, have determined that as of the end of the period covered by this Report on Form 10-K, the disclosure controls and procedures were effective to provide reasonable assurance that such information is accumulated and communicated to our management, including our principal executive and financial officers, as appropriate to allow timely decisions regarding disclosures.
Changes in Internal Controls
There were no changes in our internal controls over financial reporting as defined in Rule 13a-15(f) or Rule 15d-15(f) under the Exchange Act that occurred during the most recent fiscal quarter that have materially affected, or are reasonably likely to materially affect, the internal controls over financial reporting.
Limitations on the Effectiveness of Controls
We believe that a control system, no matter how well designed and operated, cannot provide absolute assurance that the objectives of the control systems are met, and no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within a company have been detected.
Management’s Annual Report on Internal Control Over Financial Reporting. Our management is responsible for establishing and maintaining effective internal control over financial reporting (as defined in Rule 13a – 15(f) of the Exchange Act). There are inherent limitations to the effectiveness of any internal control, including the possibility of human error and the circumvention or overriding of controls. Accordingly, even effective internal controls can provide only reasonable assurance with respect to financial statement preparation. Further, because of changes in conditions, the effectiveness of internal control may vary over time. We have assessed the effectiveness of our internal controls over financial reporting (as defined in Rule 13a -15(f) of the Exchange Act) as of December 31, 2020. In making this assessment, we used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in "Internal Control – Integrated Framework (2013)”. Management concluded that, as of December 31, 2020, our internal control over financial reporting was effective based on the criteria established by the COSO Internal Control Framework.
This annual report does not include an attestation report of our registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by our registered public accounting firm pursuant to the rules of the Securities and Exchange Commission that permit us to provide only management’s report in this annual report.
Item 9B. Other Information.
None.
PART III
Item 10. Directors, Executive Officers, and Corporate Governance.
Background and Experience of Directors
When considering whether directors and nominees have the experience, qualifications, attributes or skills, taken as a whole, to enable the Board of Directors to satisfy its oversight responsibilities effectively in light of our business and structure, the Nominating, Governance and Compliance Committee focused primarily on each person’s background and experience as reflected in the information discussed in each of the directors’ individual biographies set forth immediately below. We believe that our directors provide an appropriate mix of experience and skills relevant to the size and nature of our business. As more specifically described in such person’s individual biographies set forth below, our directors possess relevant and industry-specific experience and knowledge in the engineering financial and business fields, as the case may be, which we believe enhances the Board’s ability to oversee, evaluate and direct our overall corporate strategy. The Nominating, Governance and Compliance Committee annually reviews and makes recommendations to the Board regarding the composition and size of the Board so that the Board consists of members with the proper expertise, skills, attributes, and personal and professional backgrounds needed by the Board, consistent with applicable regulatory requirements.
The Nominating, Governance and Compliance Committee believes that all directors, including nominees, should possess the highest personal and professional ethics, integrity, and values, and be committed to representing the long-term interests of our shareholders. The Nominating, Governance and Compliance Committee will consider criteria including the nominee’s current or recent experience as a senior executive officer, whether the nominee is independent, as that term is defined in existing independence requirements of the NASDAQ Capital Market and the Securities and Exchange Commission, the business, scientific or engineering experience currently desired on the Board, geography, the nominee’s industry experience, and the nominee’s general ability to enhance the overall composition of the Board.
The Nominating, Governance and Compliance Committee does not have a formal policy on diversity; however, in recommending directors, the Board and the Committee consider the specific background and experience of the Board members and other personal attributes in an effort to provide a diverse mix of capabilities, contributions and viewpoints which the Board believes enables it to function effectively as the Board of Directors of a company with our size and the nature of our business.
Legal Proceedings Involving Directors
None.
Board Leadership
In January 2021, the Board appointed Lawrence J. Waldman to serve as the Chairman, which separated the positions of Chairman and CEO. Mr. Waldman also continues to serve as the Lead Independent Director. The Lead Independent Director is appointed by the Board and is responsible for coordinating the activities of the independent directors and the Chief Executive Officer of the Company to set agendas for Board meetings and chair executive sessions of the independent directors. The Lead Independent Director is also responsible for meeting, from time to time, with our Compensation Committee to discuss the Chief Executive Officer’s performance.
Our Corporate Governance practices contain several features which we believe will ensure that the Board maintains effective and independent oversight of management, including the following:
|
● |
Executive sessions without management and non-independent directors present are a standing Board agenda item. Executive sessions of the independent directors are held at any time requested by an independent director and, in any event, are held in connection with at least 100% of regularly schedule Board meetings. |
|
● |
The Board regularly meets in executive session with the CEO without other members of management present. |
|
● |
All Board committee members are independent directors. The committee chairs have authority to hold executive sessions with management and non-independent directors present. |
While our Board has no formal policy with respect to separation of the positions of Chairman and CEO or with respect to whether the Chairman should be a member of management or an independent director, we believe that the creation of the position of Lead Independent Director properly facilitates better communication between the Independent Directors on the one hand and the non-Independent Directors and members of management on the other hand and leads to improved oversight and discussions by the Board as a whole. The Chief Executive Officer of the Company, Emmanuel Lakios, is tasked with the responsibility or implementing our corporate strategy, we believe he is best suited for leading discussions with input from the Lead Independent Director, at the Board level, regarding performance relative to our corporate strategy and this discussion accounts for a significant portion of the time devoted at the Board meetings.
Our Certificate of Incorporation and Bylaws provide for our Company to be managed by or under the direction of the Board of Directors. Under our Certificate of Incorporation and Bylaws, the number of directors is fixed from time to time by the Board of Directors. The Board of Directors currently consists of six members. Directors are elected for a period of one year and thereafter serve, subject to the Bylaws, until the next annual meeting at which their successors are duly elected by the shareholders.
The following table sets for the names, ages and positions with the Company of each of our directors and executive officers, as of March 24, 2021.
|
Name |
Age |
Position(s) with the Company |
|
Emmanuel Lakios |
59 |
Chief Executive Officer, President |
|
Lawrence J. Waldman |
74 |
Chairman of the Board of Directors, Lead Independent Director, Chairperson-Audit Committee |
|
Conrad J. Gunther |
74 |
Director, Chairperson-Compensation Committee |
|
Raymond A. Nielsen |
70 |
Director, Chairperson-Nominating, Governance and Compliance Committee |
|
Robert M Brill |
74 |
Director |
|
Leonard A. Rosenbaum |
75 |
Director |
|
Martin J. Teitelbaum |
70 |
Director |
|
Thomas McNeill |
58 |
Chief Financial Officer, Secretary and Treasurer |
|
Steven Aragon |
59 |
Chief Operating Officer |
|
Kevin R. Collins |
55 |
Vice President and General Manager-SDC Division |
|
Jeffrey A. Brogan |
51 |
Vice President of Sales and Marketing |
|
Maxim Shatalov |
50 |
Vice President of Engineering and Technology |
|
Karlheinz Strobl |
61 |
Vice President of Business Development |
Emmanuel Lakios
Emmanuel Lakios was appointed to President and Chief Executive Officer of the Company on January 22, 2021. Mr. Lakios joined the Company as Vice President Sales and Marketing in February 2017. Mr. Lakios has over thirty (30) years of experience serving the semiconductor, data storage and optical device industries and is the holder of several patents in the field of process equipment and device structure. From January 2015 through February 2017, Mr. Lakios was the President and Chief Executive Officer at Sensor Electronic Technology, Inc., overseeing that company’s transition from R&D to a leading global commercial UV LED supplier. From 2003 to 2011 he was the Executive Vice President of Field Operations and President and Chief Operating Officer at Imago Scientific, bringing it from pre-revenue to a commercial leadership position in the 3D atomic scale tomography field. Mr. Lakios was previously employed at Veeco Instruments Inc. from 1984 until 2003, where he held several positions, including President of the Process Equipment Group and Executive Vice President of Field Operations. He has been involved in several acquisitions and numerous product line launches. He received his BE in Mechanical Engineering with focus in Material Science from SUNY Stony Brook in 1984.
Lawrence J. Waldman
Lawrence J. Waldman was appointed a member of the Board of Directors on October 5, 2016 and currently serves as Chairman of the Board and Chairman of the Audit Committee as well as the Lead Independent Director. Mr. Waldman has over forty years of experience in public accounting. He joined First Long Island Investors LLC, an investment and wealth management firm, as a Senior Advisor in May 2016. Prior to that Mr. Waldman served as an advisor to the accounting firm of EisnerAmper LLP, where he was previously the Partner-in-Charge of Commercial Audit Practice Development for Long Island since September 2011. Prior to joining EisnerAmper LLP, Mr. Waldman was the Partner-in-Charge of Commercial Audit Practice Development for Holtz Rubenstein Reminick, LLP from July 2006 to August 2011. Mr. Waldman was the Managing Partner of the Long Island office of KPMG LLP from 1994 through 2006, the accounting firm where he began his career in 1972. Mr. Waldman serves as a director of Apyx Medical Corporation, formerly Bovie Medical Corporation, since 2011 and he is currently the Chair of the Audit Committee and Lead Independent Director of the Board. Mr. Waldman has served as a member of the Board of Directors of Northstar/RXR Metro Income Fund, a non-traded Real Estate Investment Trust, and has served as a member of its audit committee from 2014 until October of 2018. Mr. Waldman was elected to the Board of Directors of Comtech Telecommunications Corp. in August of 2015, and since December 2015, serves as Chair of its Audit Committee. Mr. Waldman is also the Chair of the Supervisory Committee of Bethpage Federal Credit Union. Mr. Waldman previously served as a member of the State University of New York's Board of Trustees and as chair of its audit committee. He also previously served as the Chairman of the Board of Trustees of the Long Island Power Authority and as Chair and a member of the finance and audit committee of its Board of Trustees. Mr. Waldman is a Certified Public Accountant. Mr. Waldman qualifies to serve as a director, Audit Committee Chairman and Lead Independent Director because of his more than 35 years’ experience in public accounting and his service on various boards.
Conrad J. Gunther
Conrad J. Gunther has served as a member of our Board of Directors since 2000. Mr. Gunther has extensive experience in mergers and acquisitions and in raising capital through both public and private means. He has been an executive officer and director of several banks, both public and private, and has served on the boards of two other public companies. Since December 2016, Mr. Gunther has served as an Executive Vice President and Chief Lending Officer for Dime Community Bank, a Long Island, New York based commercial bank, where he is responsible for all lending. From July 2015 to December 2016, Mr. Gunther served as an Executive Vice President and Senior Loan Officer for First Federal Savings Bank, a Long Island, New York based Thrift. Mr. Gunther qualifies to serve on our board of directors as a result of his experience and expertise in the financial community.
Raymond Nielsen
Raymond Nielsen was appointed a member of the Board of Directors on October 5, 2016. Mr. Nielsen was the Director of Finance for The Beechwood Organization until January 2019 and has been responsible for Project and Corporate Finance including Strategic Planning Initiatives since 2014. He has been a member of the Board of Directors of Bridgehampton National Bank and Bridge Bancorp Inc., its Parent holding company since 2013, serving on the Compensation Committee, Corporate Governance & Nominating Committee, ALCO, Loan, and the Compliance, BSA & CRA Committees. Mr. Nielsen is the former CEO of Reliance Federal Savings Bank and Herald National Bank, and a 45-year veteran of the banking industry. Mr. Nielsen also served as a Director of North Fork Bancorporation and its subsidiary North Fork Bank for 6 years where he chaired both the Compensation Committee and Audit Committee as well as having served as Lead Independent Director. Mr. Nielsen’s extensive public company, banking and real estate development experience will provide a valuable resource to the Board of Directors and Executive Management.
Robert M. Brill
Dr. Brill was appointed a Director of the Company on March 5, 2021. Dr. Brill was co-founder and managing partner of Newlight Management from 1997-2019, which managed venture capital funds that focused on early stage technology companies. Prior to co-founding Newlight, Dr. Brill was a general partner of Poly Ventures, a Long Island based venture capital fund. Dr. Brill is a member of the Board of Directors of the L.I. Angel Network, the L.I. High Tech Incubator and several private companies. Dr. Brill has also previously served on the Board of Directors of multiple public and private companies. Dr. Brill served as General Manager of Harris Corporation’s CMOS Semiconductor Division. He also held various technical and management positions at IBM’s semiconductor operation. Dr. Brill holds a Ph.D in nuclear physics from Brown University and a B.A. and a B.S. in Engineering Physics from Lehigh University. Dr. Brill had previously served on the Company’s Board from April 2018 until October 2019.
Leonard A. Rosenbaum
Leonard A. Rosenbaum founded the Company in 1982 and served as our Chairman of the Board, President and Chief Executive Officer until January 22, 2021. Mr. Rosenbaum continues as a Director. From 1971 until 1982, Mr. Rosenbaum was president, director and a principal stockholder of Nav-Tec Industries, a manufacturer of semiconductor processing equipment similar to the type of equipment we manufacture. From 1966 to 1971, Mr. Rosenbaum was employed by a division of General Instrument, a manufacturer of semiconductor materials and equipment.
Martin J. Teitelbaum, Esq.
Martin J. Teitelbaum is a director and has served as a member of our Board of Directors since 1985 and as our in-house General Counsel from May 16, 2011 until January 22, 2021. Mr. Teitelbaum is an attorney, who prior to May 16, 2011, conducted his own private practice, the Law Offices of Martin J. Teitelbaum. Prior to establishing his own firm in 1988, Mr. Teitelbaum was a partner at Guberman and Teitelbaum from 1977 to 1987. Mr. Teitelbaum earned a B.A. in Political Science from the State University of New York at Buffalo and a Juris Doctor from Brooklyn Law School. Mr. Teitelbaum has served as our outside General Counsel for many years and his legal expertise makes him an asset to the Company’s board of directors.
Thomas McNeill
Thomas McNeill was appointed as the Company’s Chief Financial Officer, Secretary and Treasurer effective as of March 4, 2019. Mr. McNeill has been a Chief Financial Officer ("CFO") since 1996 and has nineteen years' of SEC reporting experience with two public companies, as well as a full range of financial and operational experience. Since April 2015, he has been CFO at Century Direct, LLC, a printing and mailing company serving the direct mail marketing industry. From November 2014 to April 2015, he was a consultant at Mailmen Inc. until its assets were purchased by Century Direct, LLC. Mr. McNeill was CFO/COO at Nina McLemore from July 2013 to June 2014, a woman's retail apparel Company. On the Public reporting side, he was CFO at DineWise, Inc. from April 2006 to April 2013, a direct to consumer prepared frozen foods company, and from October 1996 to April 2006, was CFO at Global Payment Technologies, Inc, a hi-tech manufacturing and engineering company. Mr. McNeill is a Certified Public Accountant who began his career at KPMG, achieving the position of audit manager. Mr. McNeill holds a BBA in accounting from Hofstra University.
Steven Aragon
Dr. Steven Aragon was appointed Chief Operating Officer by the Board of Directors on October 20, 2014. Dr. Aragon has over 25 years of thin-film process, materials, and system expertise applied to photovoltaic, optical, electronic, and magnetic device fabrication. He received his Ph.D. in Physical Chemistry from the University of California, Santa Cruz, in 1990 and his MBA from Santa Clara University in 1996. He is the holder of five process equipment design patents. Dr. Aragon was a co-founder of Optimus Energy Systems International Inc. and served as its Chief Technical Officer and Senior Vice-President – Engineering from November 2011 to October 2014. From June 2008 to October 2011, He has also served as Vice-President – Engineering at Stion Corp of San Jose, California, a maker of nanostructure-based CIGS (copper indium gallium sulphur-diselenide) thin-film photovoltaic panels and as the Vice President – Engineering at Day Star Technologies Inc. from June 2001 to June 2008.
Kevin R. Collins
Prior to his appointment as Vice President and General Manager-SDC Division, Mr. Collins served as the General Manager of CVD’s SDC Division since 1999. From 1990 to 1999 he was employed by Stainless Design Corp. as Manager of Field Operations and Product Development Advisor. Mr. Collins attended Columbia University School of Engineering and Applied Science.
Jeffrey A. Brogan
Dr. Jeffrey Brogan was appointed as Vice President Sales and Marketing for CVD Equipment on March 23, 2021. Previously he was Director of Sales and Marketing for CVD Materials Corporation since November 2017 with General Management responsibilities of CVD MesoScribe Technologies Corporation. Dr. Brogan served as the President and CEO of MesoScribe Technologies, Inc., spearheading its sale to CVD in 2017. He has over 20 years of experience in strategic sales & marketing, technology management, and advanced research & development. Dr. Brogan has led the development of innovative sensor products, transitioning high performance products to manufacturing using the Company’s Direct Write MesoPlasma™ printing technology. He received his PhD in Materials Science and Engineering from Stony Brook University in 1996.
Maxim Shatalov
Dr. Shatalov joined CVD as Vice President of Engineering and Technology in April 2018. Prior to CVD Mr. Shatalov was employed by Sensor Electronic Technology Inc. (SETi) a LED company where he held multiple technical and management positions from 2006 thru 2018. In 2017 Dr. Shatalov became Vice President of Technology responsible for UV LED technology and LED application development at SETi. Dr. Shatalov has over twenty years of experience in semiconductor research and devices and holds more than 12 U.S. patents.
Karlheinz Strobl
Dr. Karlheinz Strobl has been the Vice President of Business Development since October 2007. From 1997 to 2007, he was the founder and President of eele Laboratories, LLC, a technology and manufacturing solutions development company for a novel Light Engine for the video and data projection display market. Dr. Strobl holds over 14 patents and earned an MBA from Boston University, a PhD from the University of Innsbruck and an MS from both the University of Innsbruck and the University of Padova. He has also worked at the Max Planck Institute and at Los Alamos National Laboratory.
Code Of Ethics
We have adopted a Corporate Code of Conduct and Ethics that applies to our employees, senior management and Board of Directors, including the Chief Executive Officer and Chief Financial Officer. The Corporate Code of Conduct and Ethics is available on our website, http://www.cvdequipment.com, by clicking on "About Us” and then clicking on "Corporate Overview.”
Audit Committee
Our Board of Directors has an Audit Committee that currently consists of, Lawrence J. Waldman, Chairman, Conrad J. Gunther and Raymond A. Nielsen. During the fiscal year ended December 31, 2020, the Audit Committee held six meetings. Pursuant to the Audit Committee Charter, the Audit Committee is directly responsible for the appointment, compensation, retention and oversight of the work of any independent registered public accounting firm engaged for the purpose of preparing or issuing an audit report or performing other audit, review or attest services for us, and each such independent auditor shall report directly to the Committee. The Audit Committee also reviews with management and the independent auditors, our annual audited financial statements (including the disclosures under "Management’s Discussion and Analysis of Financial Condition and Results of Operations”), the scope and results of annual audits and the audit and non-audit fees of the independent registered public accounting firm. Messrs. Gunther, Waldman and Nielsen are "independent” under the requirements of the NASDAQ Stock Market.
The Board of Directors has determined that each of Messrs. Gunther and Waldman is an "audit committee financial expert” as that term is defined in the rules and regulations of the Securities and Exchange Commission.
Section 16(a) Beneficial Ownership Reporting Compliance
The rules of the Securities and Exchange Commission require us to disclose late filings of reports of stock ownership and changes in stock ownership by our directors, officers and ten percent shareholders. To our knowledge, based solely on our review of (a) the copies of such reports and amendments thereto furnished to us and (b) written representations that no other reports were required, during our fiscal year ended December 31, 2020, all of the filings for our officers, directors and ten percent shareholders were made on a timely basis.
Item 11. Executive Compensation.
Summary Compensation Table
The following table sets forth the compensation of our chief executive officer and chief financial officer, and our "named executive officers,” for the years ended December 31, 2020 and 2019.
|
Name and principal position |
Year |
Salary ($) |
Bonus ($) |
Option Awards ($) (1) |
Stock Awards ($) (1) |
All Other Compensation |
Total ($) |
|
Leonard A. Rosenbaum President and Chief Executive Officer (4) |
2020 2019 |
310,000 310,000 |
1,000 - |
- - |
- - |
5,962(2) 11,923(2) |
316,962 321,923 |
|
Thomas McNeill (3) Secretary and Chief Financial Officer |
2020 2019 |
228,000 184,154 |
1,000 - |
- - |
- 41,500 |
- - |
229,000 225,654 |
|
Martin J. Teitelbaum General Counsel and Assistant Secretary (5) |
2020 2019 |
280,000 269,231 |
1,000 - |
- - |
- - |
- - |
281,000 269,231 |
|
(1) |
Amounts shown do not reflect compensation actually received by the named executive officer. Instead, the amounts shown reflect the total remaining compensation on restricted stock and option awards granted, that have not previously been shown, as determined pursuant to ASC 718. The assumptions used to calculate the value of stock and option awards are set forth under Note 10 of the Notes to Consolidated Financial Statements. This column represents the grant date fair value of the awards as calculated in accordance with FASB ASC 718 (Stock Compensation). Pursuant to SEC rule changes effective February 28, 2010, we are required to reflect the total grant date fair values of the option grants in the year of grant, rather than the portion of this amount that was recognized for financial statement reporting purposes in a given fiscal year which was required under the prior SEC rules, resulting in a change to the amounts reported in prior Annual Reports, which was valued utilizing the grant date fair value in the year granted. |
|
(2) |
Represents payment for accrued and unused vacation time. |
|
(3) |
Effective March 4, 2019, Thomas McNeill was appointed CFO, Secretary and Treasurer. |
|
(4) |
Effective January 22, 2021, Leonard A. Rosenbaum’s employment with the Company terminated and Emmanuel Lakios was then appointed as President and Chief Executive Officer. |
|
(5) |
Effective January 22, 2021, Martin J. Teitelbaum’s employment with the Company was terminated. |
Employment Agreements and Potential Payments Upon Termination or Change in Control
There are no arrangements for compensation of directors and there are no employment contracts between the company and its directors or any change in control arrangements.
Outstanding Equity Awards at December 31, 2020
The following table sets forth the outstanding equity awards held by our named executive officers as of December 31, 2020.
|
OPTION AWARDS |
STOCK AWARDS |
||||||||||||||||||||||||||
|
Name |
Number of Securities Underlying Options Exercisable |
Number of Securities Options Unexercisable |
Exercise Price |
Option Expiration Date |
Number of shares or units of stock that have not vested |
Market value of shares or units of stock that have not vested |
Equity Incentive Plan Awards: Number of unearned shares or units that not vested |
Equity Incentive Plan Awards: Market or payout value of unearned shares or units that have not vested |
|||||||||||||||||||
|
Leonard A. Rosenbaum (3) |
- | - | $ | - | |||||||||||||||||||||||
|
Thomas McNeill (2) |
- | 7,500 | (1) | $ | 26,675 | ||||||||||||||||||||||
|
Martin J. Teitelbaum (4) |
1,400 | $ | 7.90 |
1/15/2021 |
- | $ | - | ||||||||||||||||||||
|
(1) |
Restricted stock units vest as to 2,500 shares respectively on March 4, 2021 and 5,000 shares on March 4, 2022. |
|
(2) |
Effective March 4, 2019, Thomas McNeill was appointed CFO, Secretary and Treasurer. |
|
(3) |
Effective January 22, 2021, Mr. Rosenbaum’s employment with the Company was terminated and Emmanuel Lakios was then appointed as President and CEO. |
|
(4) |
Effective January 22, 2021, Mr. Teitelbaum’s employment with the Company was terminated. |
2020 Director Compensation
The following table sets forth a summary of the compensation we paid to our non-employee directors in 2020.
|
Fees Earned or |
Restricted Stock |
|||||||||||||||
|
Name |
Paid in Cash |
Option Awards (1) |
Awards (1) |
Total |
||||||||||||
|
Conrad J. Gunther |
$ | 22,000 | - | $ | 30,173 | $ | 52,173 | |||||||||
|
Lawrence J. Waldman |
60,000 | - | 40,901 | 100,901 | ||||||||||||
|
Raymond A. Nielsen |
22,000 | - | 30,173 | 52,173 | ||||||||||||
|
(1) |
Amounts shown do not necessarily reflect compensation actually received by the named director. Instead, the amounts shown are the compensation costs recognized by CVD in fiscal 2019 for awards as determined pursuant to ASC 718. The assumptions used to calculate the value of option awards are set forth under Note 11 of the Notes to Consolidated Financial Statements. |
On May 9, 2016, the Board of Directors adopted a Director Compensation Plan for all non-employee directors, which retroactively from January 1, 2016, provided for annual compensation of approximately fifty thousand dollars ($50,000) to each non-employee director in a combination of 40% cash and 60% stock grant.
On December 14, 2018, the Board of Directors approved a new Director Compensation Plan for all non-employee directors which is effective January 1, 2019 and provides for additional compensation to Committee Chairs as well as for the Independent Lead Director. The independent Lead Director receives $30,000 in cash, the Audit Chairman receives $25,000 in combination of cash and stock grants, and the other Committee Chairs receive amounts ranging from $5,000-$10,000 in a combination of cash and stock grants.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The following table sets forth, as of March 24, 2021, information regarding the beneficial ownership of our common stock by (a) each person who is known to us to be the owner of more than five percent (5%) of our common stock, (b) each of our directors, (c) each of the named executive officers, and (d) all directors and executive officers and executive employees as a group. For purposes of the table, a person or group of persons is deemed to have beneficial ownership of any shares that such person has the right to acquire within 60 days of March 24, 2021.
|
Name and Address of Beneficial Owner (1) |
Amounts and Nature of Beneficial Ownership (2) |
Percent of Class (%) |
|||||||
|
Leonard A. Rosenbaum |
832,168 | (3) | 12.5 | ||||||
|
Leviticus Partners, L.P. |
500,000 | 7.5 | |||||||
|
Karlheinz Strobl |
123,521 | 1.9 | |||||||
|
Steven Aragon |
108,755 | 1.6 | |||||||
|
Kevin R. Collins |
91,660 | 1.4 | |||||||
|
Emmanuel Lakios |
86,368 | (4) | 1.3 | ||||||
|
Conrad J. Gunther |
84,378 | (5) | 1.3 | ||||||
|
Martin J. Teitelbaum |
72,997 | (6) | 1.1 | ||||||
|
Lawrence J. Waldman |
45,400 | (7) | * | ||||||
|
Raymond A. Nielsen |
39,200 | (8) | * | ||||||
|
Robert M. Brill |
- | (9) | * | ||||||
|
Thomas McNeill |
5,000 | (10) | * | ||||||
|
Maxim Shatalov |
20,000 | * | |||||||
|
Jeffrey A Brogan |
24,519 | * | |||||||
|
All directors and executive officers and executive employees as a group (thirteen persons) |
1,533,966 | 23.0 | |||||||
*Less than 1% of the outstanding common stock or less than 1% of the voting power
|
(1) |
The address of Messrs. Rosenbaum, Teitelbaum, Gunther, Waldman, Nielsen, Brill, McNeill, Strobl, Aragon, Strobl, Shatalov, Lakios and Brogan is c/o CVD Equipment Corporation, 355 South Technology Drive, Central Islip, New York 11722. The address of Mr. Collins is c/o Stainless Design Concepts, 1117 Old Kings Highway, Saugerties, NY 12477. The address of Leviticus Partners, L.P. is 200 Park Avenue, Suite 1700, New York, NY 10166 |
|
(2) |
All of such shares are owned directly with sole voting and investment power, unless otherwise noted below. |
|
(3) |
Does not include 4,800 shares of unvested restricted common stock. |
|
(4) |
Does not include unvested options to purchase 20,000 shares of our common stock. |
|
(5) |
Does not include 7,100 shares of unvested restricted common stock. |
|
(6) |
Includes 2,000 shares held by Mr. Teitelbaum’s wife as to which beneficial ownership thereof is disclaimed by Mr. Teitelbaum. Does not include 4,800 shares of unvested restricted common stock. |
|
(7) |
Does not include 9,600 shares of unvested restricted common stock. |
|
(8) |
Does not include 7,100 shares of unvested restricted common stock. |
|
(9) |
Does not include 5,800 shares of unvested restricted common stock. |
|
(10) |
Does not include 5,000 shares of unvested restricted common stock. |
See Item 5, Market for Registrant’s Common Equity, Related Stockholder Matters, and Issuer Purchases of Equity Securities under the heading "Equity Compensation Plan Information” for information regarding our securities authorized for issuance under equity compensation plans.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
Transactions with related persons, promoters and certain control persons.
None.
Director Independence
The current members of our Board of Directors are Lawrence J. Waldman, Conrad J. Gunther, Raymond A. Nielsen, Robert M. Brill, Leonard A. Rosenbaum and Martin J. Teitelbaum. Messrs. Waldman, Gunther, Nielsen and Brill have been determined to be "independent” as defined under Rule 4200 of the Nasdaq Stock Market.
Item 14. Principal Accountant Fees and Services.
Effective September 20, 2019, the Company authorized the engagement of Marcum, LLP, Certified Public Accountants ("Marcum”) to serve as the Company’s independent registered public accounting firm for the fiscal year ending December 31, 2019. Marcum also performed the review of the Company’s interim quarterly period ending September 30, 2019. Previously MSPC, Certified Public Accountants and Advisors ("MSPC) were the Company’s independent registered public accounting firm. The following presents fees for professional audit services rendered by Marcum, for the year ended December 31, 2020 and 2019, and MSPC, for the first two quarters of 2019.
|
2020 |
2019 |
|||||||
|
Audit Fees |
$ | 147,500 | $ | 145,500 | ||||
|
Audit-Related Fees |
10,000 | 10,000 | ||||||
|
All Other Fees |
- | - | ||||||
|
Total Fees |
$ | 157,500 | $ | 155,500 | ||||
Audit-Fees
Audit fees for 2019 consisted of the review of the first and second quarters of 2019 by MSPC and the review of the third quarter and audit of the year-end by Marcum.
Audit -related Fees
Consisted of the audit of the Company’s Defined Contribution Plan 401(k) by Marcum.
Audit Committee Approval
The engagement of the Company’s independent registered public accounting firm is pre-approved by the Company’s Audit Committee. The Audit Committee pre-approves all fees billed and all services rendered by the Company’s independent registered public accounting firm.
PART IV
Item 15. Exhibits, Financial Statement Schedules
|
3.1 |
|
3.2 |
|
3.3 |
|
3.4 |
|
3.5 |
|
4.1 |
|
10.1 |
|
10.2 |
|
10.3 |
|
10.4 |
|
10.5 |
|
10.6 |
|
10.7 |
|
10.8 |
|
10.9 |
|
10.10 |
|
10.11 |
|
10.12 |
|
10.13 |
|
10.14 |
|
10.15 |
|
10.16 |
|
10.17 |
|
10.18 |
|
10.19 |
|
10.20 |
|
10.21 |
|
10.22 |
|
10.23 |
|
10.24 |
|
10.25 |
|
10.26 |
|
23.1 |
**Consent of MARCUM, Certified Public Accountants and Advisors, A Professional Corporation (S-8). |
|
31.1 |
**Rule 13a-14(a)/15d-14(a) Certification of Chief Executive Officer. |
|
31.2 |
**Rule 13a-14(a)/15d-14(a) Certification of Chief Financial Officer. |
|
32.1 |
**Section 1350 Certification of Principal Executive Officer. |
|
32.2 |
**Section 1350 Certification of Principal Financial Officer. |
101.INS*** XBRL Instance
101.SCH*** XBRL Taxonomy Extension Schema
101.CAL*** XBRL Taxonomy Extension Calculation
101.DEF*** XBRL Taxonomy Extension Definition
101.LAB*** XBRL Taxonomy Extension Labels
101.PRE*** XBRL Taxonomy Extension Presentation
* Management contract or compensatory plan or arrangement required
** Filed herewith
*** XBRL information is furnished and not filed or a part of a registration statement or prospectus for purposes of sections 11 or 12 of the Securities Act of 1933, as amended, is deemed not filed for purposes of section 18 of the Securities Exchange Act of 1934, as amended, and otherwise is not subject to liability under these sections.
SIGNATURES
In accordance with Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
DATE: March 31, 2021
|
CVD EQUIPMENT CORPORATION |
|
By: /s/ Emmanuel Lakios |
|
Name: Emmanuel Lakios |
|
Title: President and Chief Executive Officer |
|
By: /s/ Thomas McNeill |
|
Name: Thomas McNeill |
|
Title: Chief Financial Officer and Secretary |
|
Principal Financial and Accounting Officer |
In accordance with the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated below.
|
NAME |
POSITION |
DATE |
|
/s/ Emmanuel Lakios |
President, Chief Executive Officer |
3/31/2021 |
|
Emmanuel Lakios |
(Principal Executive Officer) |
|
|
/s/ Lawrence J. Waldman |
Director, Chairman of the Board |
3/31/2021 |
|
Lawrence J. Waldman |
||
|
/s/ Conrad J. Gunther |
Director |
3/31/2021 |
|
Conrad J. Gunther |
||
|
/s/ Raymond A. Nielsen |
Director |
3/31/2021 |
|
Raymond A. Nielsen |
||
|
/s/ Robert M. Brill |
Director |
3/31/2021 |
|
Robert M. Brill |
||
|
/s/ Leonard A Rosenbaum |
Director |
3/31/2021 |
|
Leonard A. Rosenbaum |
||
|
/s/ Martin J. Teitelbaum |
Director |
3/31/2021 |
|
Martin J. Teitelbaum |
CVD EQUIPMENT CORPORATION AND SUBSIDIARIES
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
|
Page No. |
|
|
Report of Independent Registered Public Accounting Firms |
F-1 |
|
Financial Statements: |
|
|
Consolidated Balance Sheets as of December 31, 2020 and 2019 |
F-3 |
|
Consolidated Statements of Operations for the years ended December 31, 2020 and 2019 |
F-4 |
|
Consolidated Statements of Changes in Stockholders’ Equity for the years ended December 31, 2020 and 2019 |
F-5 |
|
Consolidated Statements of Cash Flows for the years ended December 31, 2020 and 2019 |
F-6 |
|
Notes to Consolidated Financial Statements |
F-7 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Board of Directors of
CVD Equipment Corporation and Subsidiaries
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of CVD Equipment Corporation and Subsidiaries (the "Company”) as of December 31, 2020 and 2019, the related consolidated statements of operations, stockholders’ equity and cash flows for each of the two years in the period ended December 31, 2020, and the related notes (collectively referred to as the "financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2020 and 2019, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2020, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Revenue Recognition – Estimated Total Contract Costs
Description of the Matter
As discussed in Notes 2 and 3 to the consolidated financial statements, the Company recognizes systems revenue over time by using an input method based on costs incurred as it best depicts the Company’s progress toward satisfaction of the performance obligation. Under this method, revenue arising from contracts is recognized as work is performed based on the ratio of costs incurred to date to the total estimated costs at completion of the performance obligations. The estimation of these costs requires judgment by the Company given the unique product specifications and requirements for contracts related to the design, development, and manufacture of the system. During the year ended December 31, 2020, the Company recognized approximately $6.7 million of revenue recognized over time.
Subjective judgment is required by management in determining the assumptions in estimating the estimated costs to complete on contracts for which revenue is recognized over time using a cost-to-cost model. Complex auditor judgment was required in evaluating initial cost estimates and expected costs to complete.
How We Addressed the Matter in Our Audit
The primary procedures we performed to address this critical audit matter included the following:
|
● |
Obtaining an understanding of management’s process in developing the cost estimates; |
|
● |
Evaluating management’s ability to reasonably estimate costs by performing a comparison of the actual costs to prior period estimates, including evaluating the timely identification of circumstances that may warrant a modification to the estimated costs; |
|
● |
Evaluate management’s methodologies and the consistency of management’s methodologies over the life of the contracts; |
|
● |
Tested the original estimated costs and profit margins on System projects that were commenced and completed during the year ending December 31, 2020, by obtaining the original estimates, compare to the actual costs and profit margin for the completed contracts and investigate significant changes; and |
|
● |
Tested the estimated costs to complete Systems projects that were not completed during the year ended December 31, 2020 by comparing the estimated cost to complete at December 31, 2020 to actual cost incurred subsequent to December 31, 2020. |
/s/ Marcum llp
Marcum llp
We have served as the Company’s auditor since 2019.
Melville, NY
March 31, 2021
CVD EQUIPMENT CORPORATION AND SUBSIDIARIES
Consolidated Balance Sheets
As of December 31, 2020 and 2019
|
December 31, 2020 |
December 31, 2019 |
|||||||
|
ASSETS |
||||||||
|
Current Assets |
||||||||
|
Cash and cash equivalents |
$ | 7,699,335 | $ | 8,664,253 | ||||
|
Accounts receivable, net |
1,047,728 | 2,545,537 | ||||||
|
Contract assets |
494,281 | 512,952 | ||||||
|
Inventories, net |
1,123,839 | 1,709,713 | ||||||
|
Taxes receivable |
715,599 | - | ||||||
|
Other current assets |
709,175 | 733,337 | ||||||
|
Total Current Assets |
11,789,957 | 14,165,792 | ||||||
|
Property, plant and equipment, net |
28,843,563 | 32,102,335 | ||||||
|
Other assets |
13,748 | 13,748 | ||||||
|
Intangible assets, net |
288,657 | 441,177 | ||||||
|
Total Assets |
$ | 40,935,925 | $ | 46,723,052 | ||||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY |
||||||||
|
Current Liabilities |
||||||||
|
Accounts payable |
$ | 817,933 | $ | 535,394 | ||||
|
Accrued expenses |
1,409,039 | 1,902,858 | ||||||
|
Current maturities of long-term debt |
690,667 | 674,593 | ||||||
|
Contract liabilities |
786,657 | 2,275,236 | ||||||
|
Total Current Liabilities |
3,704,296 | 5,388,081 | ||||||
|
Long-term debt, net of current portion |
13,106,057 | 11,377,126 | ||||||
|
Total Liabilities |
16,810,353 | 16,765,207 | ||||||
|
Commitments and contingencies (see note 14) |
||||||||
|
Stockholders’ Equity: |
||||||||
|
Common stock - $0.01 par value – 20,000,000 shares authorized; issued and outstanding 6,678,698 at December 31, 2020 and 6,623,793 at December 31, 2019 |
66,786 | 66,237 | ||||||
|
Additional paid-in capital |
26,961,684 | 26,719,554 | ||||||
|
(Accumulated deficit) / Retained earnings |
(2,902,898 | ) | 3,172,054 | |||||
|
Total Stockholders’ Equity |
24,125,572 | 29,957,845 | ||||||
|
Total Liabilities and Stockholders’ Equity |
$ | 40,935,925 | $ | 46,723,052 | ||||
The accompanying notes are an integral part of the consolidated financial statements
CVD EQUIPMENT CORPORATION AND SUBSIDIARIES
Consolidated Statements of Operations
Years ended December 31, 2020 and 2019
|
2020 |
2019 |
|||||||
|
Revenue |
$ | 16,920,219 | $ | 19,646,652 | ||||
|
Cost of revenue |
14,037,813 | 16,850,077 | ||||||
|
Gross profit |
2,882,406 | 2,796,575 | ||||||
|
Operating expenses |
||||||||
|
Research and development |
372,648 | 597,456 | ||||||
|
Selling and shipping |
580,468 | 898,338 | ||||||
|
Impairment charge |
3,599,322 | - | ||||||
|
General and administrative |
6,153,925 | 6,285,496 | ||||||
|
Total operating expenses |
10,706,363 | 7,781,290 | ||||||
|
Operating loss |
(7,823,957 | ) | (4,984,715 | ) | ||||
|
Other income (expense): |
||||||||
|
Interest income |
62,667 | 142,579 | ||||||
|
Interest expense |
(444,337 | ) | (482,844 | ) | ||||
|
Other income |
603,320 | 411,230 | ||||||
|
Total other income, net |
221,650 | 70,965 | ||||||
|
Loss before income tax |
(7,602,307 | ) | (4,913,750 | ) | ||||
|
Income tax (benefit) expense |
(1,527,355 | ) | 1,413,908 | |||||
|
Net loss |
$ | (6,074,952 | ) | $ | (6,327,658 | ) | ||
|
Basic loss per common share |
$ | (0.91 | ) | $ | (0.96 | ) | ||
|
Diluted loss per common share |
$ | (0.91 | ) | $ | (0.96 | ) | ||
|
Weighted average common shares |
||||||||
|
Outstanding-basic |
6,640,272 | 6,562,141 | ||||||
|
Weighted average common shares |
||||||||
|
Outstanding-diluted |
6,640,272 | 6,562,141 | ||||||
The accompanying notes are an integral part of the consolidated financial statements
CVD EQUIPMENT CORPORATION AND SUBSIDIARIES
Consolidated Statements of Changes in Stockholders’ Equity
|
Years ended December 31, 2020 and 2019 |
||||||||||||||||||||
|
Common stock |
||||||||||||||||||||
|
Shares |
Par Value |
Additional paid-in Capital |
Retained Earnings / (Accumulated Deficit) |
Total |
||||||||||||||||
|
Balance at January 1, 2019 |
6,535,888 | $ | 65,358 | $ | 26,148,256 | $ | 9,499,712 | $ | 35,713,326 | |||||||||||
|
Net loss |
- | - | - | (6,327,658 | ) | (6,327,658 | ) | |||||||||||||
|
Share-Based Compensation |
87,905 | 879 | 571,298 | - | 572,177 | |||||||||||||||
|
Balance at December 31, 2019 |
6,623,793 | $ | 66,237 | $ | 26,719,554 | $ | 3,172,054 | $ | 29,957,845 | |||||||||||
|
Net loss |
- | - | - | (6,074,952 | ) | (6,074,952 | ) | |||||||||||||
|
Share-Based Compensation |
54,905 | 549 | 242,130 | - | 242,679 | |||||||||||||||
|
Balance at December 31, 2020 |
6,678,698 | 66,786 | 26,961,684 | (2,902,898 | ) | 24,125,572 | ||||||||||||||
The accompanying notes are an integral part of the consolidated financial statements
CVD EQUIPMENT CORPORATION AND SUBSIDIARIES
Consolidated Statements of Cash Flows
Years ended December 31, 2020 and 2019
|
2020 |
2019 |
|||||||
|
Cash flows from operating activities: |
||||||||
|
Net loss |
$ | (6,074,952 | ) | $ | (6,327,658 | ) | ||
|
Adjustments to reconcile net loss to net cash (used in) provided by operating activities |
||||||||
|
Impairment charge |
3,599,322 | - | ||||||
|
Stock-based compensation |
242,679 | 572,177 | ||||||
|
Depreciation and amortization |
1,389,145 | 1,042,829 | ||||||
|
Deferred income tax benefit |
- | 1,425,414 | ||||||
|
Recovery on contingent earnout |
- | (200,000 | ) | |||||
|
Bad debt expense |
140,044 | - | ||||||
|
(Increase)/decrease in operating assets |
||||||||
|
Accounts receivable |
1,357,765 | 1,519,683 | ||||||
|
Contract assets |
18,671 | 844,846 | ||||||
|
Inventories |
585,874 | 152,160 | ||||||
|
Tax receivable |
(715,599 | ) | - | |||||
|
Other current assets |
24,162 | - | ||||||
|
Other assets |
- | 125,056 | ||||||
|
Increase/(decrease) in operating liabilities |
||||||||
|
Accounts payable |
282,539 | (177,801 | ) | |||||
|
Accrued expenses |
(493,819 | ) | 315,195 | |||||
|
Contract liabilities |
(1,488,579 | ) | 1,278,813 | |||||
|
Total adjustments |
4,942,204 | 6,898,372 | ||||||
|
Net cash (used in) provided by operating activities |
(1,132,748 | ) | 570,714 | |||||
|
Cash flows from investing activities: |
||||||||
|
Capital expenditures |
(1,577,175 | ) | (2,688,231 | ) | ||||
|
Net cash used in investing activities |
(1,577,175 | ) | (2,688,231 | ) | ||||
|
Cash flows from financing activities |
||||||||
|
Proceeds from Payroll Protection Plan Loan |
2,415,970 | - | ||||||
|
Payments of long-term debt |
(670,965 | ) | (657,591 | ) | ||||
|
Net cash provided by (used) in financing activities |
1,745,005 | (657,591 | ) | |||||
|
Net decrease in cash and cash equivalents |
(964,918 | ) | (2,775,108 | ) | ||||
|
Cash and cash equivalents at beginning of period |
8,664,253 | 11,439,361 | ||||||
|
Cash and cash equivalents at end of period |
$ | 7,699,335 | $ | 8,664,253 | ||||
|
Supplemental disclosure of cash flow information: |
||||||||
|
Income taxes paid |
$ | 3,040 | $ | 2,800 | ||||
|
Interest paid |
$ | 445,109 | $ | 365,254 | ||||
|
Supplemental disclosure of non-cash investing and financing activities: |
||||||||
|
Capitalization of right to use Asset |
$ | - | $ | 84,354 | ||||
The accompanying notes are an integral part of the consolidated financial statements
CVD EQUIPMENT CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
Note 1 – Business Description
CVD Equipment Corporation and its subsidiaries (the "Company”), a New York corporation, was organized and commenced operations in October 1982. Its principal business activities include the manufacturing of chemical vapor deposition equipment, customized gas control systems, the manufacturing of process equipment suitable for the synthesis of a variety of one-dimensional nanostructures and nanomaterials and a line of furnaces, all of which are used primarily to produce semiconductors and other electronic components. The Company engages in business throughout the United States and internationally.
Note 2 - Summary of Significant Accounting Policies
Principles of Consolidation
The consolidated financial statements include the accounts of CVD Equipment Corporation and its wholly owned subsidiaries. The Company has five wholly owned subsidiaries: CVD Materials Corporation, which provides material coatings, process development support and process startup assistance through Tantaline ApS and CVD MesoScribe Technologies Corporation, FAE Holdings 411519R, LLC, a real estate holding company whose sole asset is its interest in the real estate and building housing our corporate headquarters and 555 N Research Corporation whose sole asset is its interest in the real estate and building located at 555 North Research Place, Central Islip, NY. All significant intercompany accounts and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
The Company’s significant estimates are the accounting for certain items such as revenues on long-term contracts recognized on the input method, depreciation and amortization, valuation of inventories at the lower of cost or net realizable value; allowance for doubtful accounts receivable; valuation allowances for deferred tax assets, impairment considerations of long-lived assets and valuation of stock-based compensation.
Reclassification
Certain reclassifications have been made to prior year amounts to conform with the current year presentation.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
Note 2 - Summary of Significant Accounting Policies (continued)
Revenue Recognition
The Company designs, manufactures and sells custom chemical vapor deposition equipment through contractual agreements. These system sales require the Company to deliver functioning equipment that is generally completed within three to eighteen months from commencement of order acceptance. The Company recognizes revenue over time by using an input method based on costs incurred as it depicts the Company’s progress toward satisfaction of the performance obligation. Under this method, revenue arising from fixed price contracts is recognized as work is performed based on the ratio of costs incurred to date to the total estimated costs at completion of the performance obligations, typically within three months to eighteen months.
Incurred costs include all direct material and labor costs and those indirect costs related to contract performance, such as supplies, tools, repairs and depreciation costs. Contract material costs are included in incurred costs when the project materials have been purchased or moved to work in process, and installed, as required by the project’s engineering design. Cost based input methods of revenue recognition require the Company to make estimates of costs to complete the projects. In making such estimates, significant judgment is required to evaluate assumptions related to the costs to complete the projects, including materials, labor and other system costs. If the estimated total costs on any contract are greater than the net contract revenues, the Company recognizes the entire estimated loss in the period the loss becomes known and can be reasonably estimated.
"Contract assets,” include unbilled amounts typically resulting from system sales under contracts and revenue recognition exceeds the amount billed to the customer. The amount may not exceed their estimated net realizable value. Contract assets are classified as current based on our contract operating cycle.
"Contract liabilities,” include advance payments and billings in excess of revenue recognized. The Company typically receives down payments upon receipt of order and progress payments during the manufacturing cycle. Contract liabilities are classified as current based on our contract operating cycle and reported on a contract-by-contract basis, net of revenue recognized, at the end of each reporting period
For outright sales of products, revenue is recognized when control of the promised products or services is transferred to our customers, in an amount that reflects the consideration we expect to be entitled to in exchange for those products or services (the transaction price). A performance obligation is a promise in a contract to transfer a distinct product or service to a customer and is the unit of account under ASC 606 ("Revenue from Contracts with Customers”).
Inventories
Inventories are valued at the lower of cost (determined on the first-in, first-out method) or net realizable value.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
Note 2 - Summary of Significant Accounting Policies (continued)
Income Taxes
Deferred tax assets and liabilities are determined based on the estimated future tax effects of temporary differences between the financial statements and tax bases of assets and liabilities, as measured by using the future enacted tax rates. Deferred tax expense (benefit) is the result of changes in the deferred tax assets and liabilities. The Company records a valuation allowance against deferred tax assets when it is more likely than not that future tax benefits will not be utilized based on a lack of sufficient positive evidence.
Investment tax credits are accounted for by the flow-through method, reducing income taxes currently payable and the provision for income taxes in the period the assets giving rise to such credits are placed in service. To the extent such credits are not currently utilized on the Company’s
tax return, deferred tax assets, subject to considerations about the need for a valuation allowance, are recognized for the carryforward amount.
The Company recognizes the tax benefit from an uncertain tax position only if it is more-likely-than-not that the tax position will be sustained on examination by taxing authorities, based on the technical merits of the position. The tax benefits recognized in the financial statements from such
positions are measured based on the largest benefit that has a greater than 50% likelihood of being realized upon ultimate settlement.
The accounting guidance on accounting for uncertainty in income taxes also addresses derecognition, classification, interest and penalties on income taxes, and accounting in interim periods. The Company does not believe it has any uncertain tax positions through the year ending December 31, 2020 which would have a material impact on the Company’s consolidated financial statements.
The Company and its subsidiaries file combined income tax returns in the U.S. Federal and New York State jurisdiction. In addition, the parent company files standalone tax returns in California, Delaware, Florida, Michigan, Minnesota, New Hampshire and Wisconsin. The Company is no longer subject to U.S. federal and state income tax examinations for tax periods before 2016.
Impairment of Long Lived Assets and Intangibles
Long-lived assets consist primarily of property, plant, and equipment. Intangibles consist of patents, copyrights and intellectual property, licensing agreements and certifications. Long-lived assets are reviewed for impairment whenever events or circumstances indicate their carrying value may not be recoverable. When such events or circumstances arise, an estimate of the future undiscounted cash flows produced by the asset, or the appropriate grouping of assets, is compared to the asset’s carrying value to determine if impairment exists pursuant to the requirements of the Financial Accounting Standards Board ("FASB”) Accounting Standards Codification ("ASC”) 360-10-35, "Impairment or Disposal of Long-Lived Assets.” If the asset is determined to be impaired, the impairment loss is measured on the excess of its carrying value over its fair value. Assets to be disposed of are reported at the lower of their carrying value or net realizable value. Based upon continued operating losses and negative cash flows from the Tantaline product line and the Company’s updated forecasting, the expected future cash flows of the Tantaline product line is negative and thus management has recorded an impairment charge of $3.6 million in the fourth quarter and year ended December 31, 2020. The Company had no recorded impairment charges in the consolidated statement of operations during the year ended December 31, 2019.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
Note 2 - Summary of Significant Accounting Policies (continued)
Property, Plant and Equipment
Property, plant and equipment are recorded at cost. Depreciation is determined on a straight-line basis for buildings and building improvements over 5 to 39 years and for machinery and equipment over 5 to 8 years. Depreciation and amortization of assets used in manufacturing are recorded in Cost of revenue. Depreciation and amortization of all other assets are recorded in Operating Expenses-General and Administrative.
Intangible Assets
The cost of intangible assets is being amortized on a straight-line basis over their estimated initial useful lives which ranged from 5 to 20 years. Amortization expense recorded by the Company in 2020 and 2019 totaled $124,550 and $120,488, respectively.
Research & Development
Research and development costs are expensed as incurred. Our laboratory staff conducts research and development independent of customer orders. For the year ended December 31 2020 and 2019, we incurred approximately $373,000 and $598,000, respectively, of research and development expenses.
Product Warranty
The Company records warranty costs as incurred and does not provide for possible future costs. Management estimates such costs are immaterial, based on historical experience.
Earnings Per Share
Basic earnings per common share is computed by dividing the net income by the weighted average number of shares of common stock outstanding during each period. When applicable, diluted earnings per common share is determined using the weighted-average number of common shares outstanding during the period, adjusted for the dilutive effect of common stock equivalents, consisting of shares that might be adjusted upon exercise of common stock options and warrants.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
Note 2 - Summary of Significant Accounting Policies (continued)
Potential common shares issued are calculated using the treasury stock method, which recognizes the use of proceeds that could be obtained upon the exercise of options and warrants in computing diluted earnings per share. It assumes that any proceeds would be used to purchase common stock at the average market price of the common stock during the period.
Cash and Cash Equivalents
The Company had cash and cash equivalents of $7.7 million and $8.7 million at December 31, 2020 and 2019, respectively. The Company invests excess cash in treasury bills, certificates of deposit or money market accounts, all with maturities of less than three months. Cash equivalents were $1.0 million and $2.1 million for the years ended December 31, 2020 and December 31, 2019, respectively.
The Company places most of its temporary cash investments with financial institutions, which from time to time may exceed the Federal Deposit Insurance Corporation limit. The amount at risk at December 31, 2020 and at December 31, 2019 was $5,822,000 and $5,198,000 respectively.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of cash, cash equivalents, and accounts receivable. The Company places its cash equivalents with financial institutions and invests its excess cash primarily in treasury bills, certificates of deposit or money market instruments. The Company has established guidelines relative to credit ratings and maturities that seek to maintain stability and liquidity.
The Company sells products and services to various companies across several industries in the ordinary course of business. The Company routinely assesses the financial strength of its customers and maintains allowances for anticipated losses based upon historical experience.
Accounts Receivable
The Company sells products and services to various companies across several industries in the ordinary course of business. The Company performs ongoing credit evaluations to assess the probability of accounts receivable collection based on a number of factors, including past
transaction experience, evaluation of their credit history and review of the invoicing terms of the contract to determine the financial strength of its customers. The Company has accounts receivables from certain customers that exceed 10%. As of December 31, 2020, and 2019, the accounts receivable balance includes amounts from two customers, which totals 35% and three customers which total 61%, respectively.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
Note 2 - Summary of Significant Accounting Policies (continued)
Accounts receivable is presented net of an allowance for doubtful accounts of $164,000 and $24,000 as of December 31, 2020 and 2019, respectively. The allowance is based on historical experience and management’s evaluation of the collectability of accounts receivable. Management believes the allowance is adequate. However, future estimates may fluctuate based on changes in economic and customer conditions. The Company doesn’t require collateral from its customers.
Sales Concentrations
Revenue to a single customer in any one year can exceed 10.0% of our total sales. Two customers represented 30.5% and two customers represented 39.3%, respectively, of our annual revenues in fiscal years 2020 and 2019. We believe that our relationships with these customers are positive and may provide us with ongoing continuous sustainability for years to come, however the loss of a large customer would have to be replaced by others, and our inability to do so may have a material adverse effect on our business and financial condition.
Export sales to customers represented approximately 16.8% and 21.3% of sales for the years ended December 31, 2020 and 2019, respectively. Export sales in both 2020 and 2019 were primarily to customers in Europe and Asia. Primarily all contracts except those entered into by CVD Tantaline ApS are denominated in U.S. dollars. The Company has not entered into any foreign exchange contracts.
Fair Value of Financial Instruments
The carrying amounts of financial instruments including cash and cash equivalents, accounts receivable, net, accounts payable, contract liabilities and customer deposits approximate fair value due to the relatively short-term maturity of these instruments. The carrying value of long-term debt approximates fair value based on prevailing borrowing rates currently available for loans with similar terms and maturities.
Stock-Based Compensation
The Company records stock-based compensation in accordance with the provisions set forth in ASC 718, "Stock Compensation”. ASC 718 requires companies to recognize the cost of employee services received in exchange for awards of equity instruments based upon the grant date fair value of those awards over the vesting period.
Shipping and Handling
It is the Company’s policy to include freight charges billed to customers in total revenue. The amount included in revenue was $6,000 and $39,000 for the years ended December 31, 2020 and 2019, respectively.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
Note 2 - Summary of Significant Accounting Policies (continued)
Liquidity and Management’s Plan
The Company has incurred recurring losses since 2018, which have resulted in an accumulated deficit of $2.9 million as of December 31, 2020. For the year ended December 31, 2020, the Company incurred a net loss of $6.1 million, which includes a $3.6 million impairment charge related to its Tantaline product line. At December 31, 2020, the Company’s cash and cash equivalents were $7.7 million, and our working capital was $8.1 million.
The Company’s current capital resources include cash and cash equivalents ($7.7 million), accounts receivable ($1.0 million), contract assets ($.5 million), inventories ($1.1 million) and a tax receivable ($.7 million). In addition, the Company receives advance deposits on new system orders.
In February 2021, the Company initiated a plan approved by the Board of directors to sell its building located at 555 North Research Place, Central Islip, NY, and to consolidate that facility into its building located at 355 South Technology Drive, Central Islip, NY, which houses manufacturing and executive offices. This significant action will monetize a substantial portion of the Company’s long-term assets, generating additional cash proceeds. In addition, the consolidation of the facilities will help lower operating costs (See Note 14).
Based upon all the above factors, including the Board approved management plan, utilizing our current operating assets and reducing other operating expenses, the Company estimates that it will have sufficient cash and cash equivalents to fund our operations for the next twelve months from the date of the filing of this annual report.
Recently Issued Accounting Standards
In June 2016, the FASB issued Accounting Standard Update ("ASU”) 2016-13, Financial Instruments – Credit Losses (Topic 326), which require that financial assets measured at amortized cost be presented at the net amount expected to be collected. The allowance for credit losses is a valuation account that is deducted from the amortized cost basis of the financial asset to present the net carrying value at the amount expected to be collected. The income statement reflects the measurement of credit losses for newly recognized financial assets, as well as the increase or decreases of expected credit losses that have taken place during the period. The measurement of expected credit losses is based upon historical experience, current conditions, and reasonable and supportable forecasts that affect the collectability of the reported amount. On November 15, 2019, the FASB delayed the effective date for smaller reporting companies. The amendments in this update are now effective for fiscal years beginning after December 15, 2022 and interim periods within those annual periods. Early adoption for fiscal years beginning after December 15, 2018 is permitted. We are currently evaluating the effect of this update on our consolidated financial statements.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
Note 2 - Summary of Significant Accounting Policies (continued)
In December 2019, the FASB issued ASU 2019-12, "Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes," which is intended to enhance and simplify various aspects of the accounting for income taxes. The amendments in this update remove certain exceptions to the general principles in Topic 740 related to the approach for intraperiod tax allocation, the methodology for calculating income taxes in an interim period and the recognition of deferred tax liabilities for outside basis differences. ASU 2019-12 also clarifies and amends existing guidance to improve consistent application of the accounting for franchise taxes, enacted changes in tax laws or rates and transactions that result in a step-up in the tax basis of goodwill. ASU 2019-12 is effective for annual and interim periods beginning after December 15, 2020, with early adoption permitted in any interim period. We believe our adoption of ASU 2019-12 in our first quarter of 2021 will not have a material effect on our consolidated financial statements.
We believe there is no additional new accounting guidance adopted, but not yet effective that is relevant to the readers of our financial statements. However, there are numerous new proposals under development which, if and when enacted, may have a significant impact on our financial reporting.
Note 3 – Revenue
The following table represents a disaggregation of revenue from contracts for the years ended December 31, 2020 and December 31, 2019:
|
Year Ending December 31, 2020 |
||||||||||||
|
Over time |
Point in time |
Total |
||||||||||
|
Aerospace |
$ | 1,607 | $ | 6,013 | $ | 7,620 | ||||||
|
Industrial |
$ | 1,849 | $ | 3,565 | $ | 5,414 | ||||||
|
Research |
$ | 3,208 | $ | 678 | $ | 3,886 | ||||||
|
Total |
$ | 6,664 | $ | 10,256 | $ | 16,920 | ||||||
|
Year ending December 31, 2019 |
||||||||||||
|
Over time |
Point in time |
Total |
||||||||||
|
Aerospace |
$ | 3,224 | $ | 5,948 | $ | 9,172 | ||||||
|
Industrial |
$ | 3,199 | $ | 3,994 | $ | 7,193 | ||||||
|
Research |
$ | 2,609 | $ | 673 | $ | 3,282 | ||||||
|
Total |
$ | 9,032 | $ | 10,615 | $ | 19,647 | ||||||
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
Note 3 – Revenue (continued)
The Company has unrecognized contract revenue of approximately $2.1 million at December 31, 2020, which it expects to recognize as revenue within the next twelve months.
Judgment is required to evaluate assumptions including the amount of net contract revenues and the total estimated costs to determine our progress towards contract completion and to calculate the corresponding amount of revenue to recognize.
Changes in estimates for sales of systems occur for a variety of reasons, including but not limited to (i) build accelerations or delays, (ii) product cost forecast changes, (iii) cost related change orders or add-ons, or (iv) changes in other information used to estimate costs. Changes in estimates may have a material effect on the Company’s consolidated statements of operations.
Contract Assets and Liabilities
Contract assets consist of (i) retainage which represent the earned, but unbilled, portion for which payment is deferred by the customer until certain contractual milestones are met; and (ii) unbilled receivables which represent revenue that has been recognized in advance of billing the customer, which is common for long-term contracts. Contract liabilities consist of customer advances and billings in excess of revenue recognized.
As of December 31, 2020, 2019 and January 1 2019, contract assets were $.5 million, $.5 million and $1.4 million, respectively. At December 31, 2020, 2019 and January 1, 2019, contract liabilities were $.2 million, $.8 million and $.5 million, respectively, and the ending balance of the contract liabilities are generally recognized as income in the following year.
Contract assets and contract liabilities on input method type contracts in progress are summarized as follows:
|
2020 |
2019 |
|||||||
|
Costs incurred on contracts in progress |
$ | 4,464,471 | $ | 6,943,066 | ||||
|
Estimated earnings |
2,087,396 | 3,370,032 | ||||||
| 6,551,867 | 10,313,098 | |||||||
|
Billings to date |
(6,212,229 | ) | (10,645,800 | ) | ||||
| $ | 339,638 | $ | (332,702 | ) | ||||
|
Deferred revenue related to non-systems contracts |
(632,014 | ) | (1,429,582 | ) | ||||
| (292,376 | ) | $ | (1,762,284 | ) | ||||
|
Included in accompanying balance sheets |
||||||||
|
Under the following captions: |
||||||||
|
Contract assets |
$ | 494,281 | $ | 512,952 | ||||
|
Contract liabilities |
$ | (786,657 | ) | $ | (2,275,236 | ) | ||
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
Note 4 - Inventories
|
Inventories consist of: |
||||||||
|
2020 |
2019 |
|||||||
|
Raw materials |
$ | 928,221 | $ | 1,281,250 | ||||
|
Work-in-process |
195,618 | 428,463 | ||||||
|
Inventories |
$ | 1,123,839 | $ | 1,709,713 | ||||
Note 5 – Property, Plant and Equipment
Major classes of property, plant and equipment consist of the following:
|
2020 |
2019 |
|||||||
|
Land |
$ | 6,929,000 | $ | 6,929,000 | ||||
|
Buildings |
15,917,000 | 15,917,000 | ||||||
|
Building improvements |
8,141,791 | 7,864,757 | ||||||
|
Machinery and equipment |
3,340,005 | 3,592,351 | ||||||
|
Furniture and fixtures |
613,765 | 613,765 | ||||||
|
Computer equipment |
493,349 | 487,902 | ||||||
|
Software |
435,593 | 438,391 | ||||||
|
Transportation equipment |
114,511 | 114,511 | ||||||
|
Lab equipment |
1,992,179 | 1,985,179 | ||||||
|
Construction in Progress |
93,936 | 2,110,875 | ||||||
|
Totals at cost |
$ | 38,071,129 | $ | 40,053,731 | ||||
|
Less: Accumulated depreciation and amortization |
(9,227,566 | ) | (7,951,396 | ) | ||||
|
Property, plant and equipment, net |
$ | 28,843,563 | $ | 32,102,335 | ||||
|
Depreciation and amortization expense (1) |
$ | 1,389,145 | $ | 1,042,829 | ||||
|
(1) Includes amortization expense of $124,550 and $120,488 for the year ending December 31, 2020 and the year ended December 31, 2019, respectively. Such amortization expense relates to other capitalized and intangibles assets. |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
Note 6 – Intangible Assets
Intangible assets consisted of the following:
|
2020 |
||||||||||||
|
Intangible Assets |
Cost |
Accumulated Amortization |
Carrying Amount |
|||||||||
|
Patents, Copyrights and Intellectual Property |
$ | 792,821 | $ | 515,665 | $ | 277,156 | ||||||
|
Licensing Agreement |
10,000 | 10,000 | 0 | |||||||||
|
Certifications |
85,032 | 73,531 | 11,501 | |||||||||
|
Totals |
$ | 887,853 | $ | 599,196 | $ | 288,657 | ||||||
|
2019 |
||||||||||||
|
Intangible Assets |
Cost |
Accumulated Amortization |
Carrying Amount |
|||||||||
|
Patents, Copyrights and Intellectual Property |
$ | 836,544 | $ | 413,957 | $ | 422,587 | ||||||
|
Licensing Agreement |
10,000 | 10,000 | 0 | |||||||||
|
Certifications |
83,272 | 64,682 | 18,590 | |||||||||
|
Totals |
$ | 929,816 | $ | 488,639 | $ | 441,177 | ||||||
The estimated amortization expense related to intangible assets for each of the five succeeding fiscal years and thereafter as of December 31, 2020 is as follows:
|
Year Ended |
||||
|
2021 |
$ | 108,344 | ||
|
2022 |
16,852 | |||
|
2023 |
14,199 | |||
|
2024 |
10,658 | |||
|
2025 |
10,658 | |||
|
Thereafter |
127,946 | |||
|
Total |
$ | 288,657 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
Note 7 – Long-term Debt
|
Long-term debt as of December 31 consists of the following: |
||||||||
|
2020 |
2019 |
|||||||
|
HSBC $10,387,500 Mortgage payable secured by real property Buildings and improvements at 555 N Research Drive, Central Islip, NY payable in monthly principal installments of $62,481 including Interest at a rate of 3.9148% maturing on December 1, 2022. |
$ | 9,315,246 | $ | 9,686,211 | ||||
|
PPP Loan $2,415,970, maturing on April 21, 2022, with interest accruing at 1% per annum |
2,415,970 | - | ||||||
|
HSBC $6,000,000 Mortgage payable secured by building Buildings and improvements at 355 South Technology Drive, Central Islip, NY payable in monthly principal installments of $25,000 plus interest. Interest presently accrues at our option, at the variable rate of LIBOR plus 1.75% or HSBC’s Prime rate minus 0.50% The loan matures on March 1, 2022. |
2,065,508 | 2,365,508 | ||||||
|
Total long-term debt |
$ | 13,796,724 | $ | 12,051,719 | ||||
|
Less: Current maturities |
(690,667 | ) | (674,593 | ) | ||||
|
Long-term debt |
$ | 13,106,057 | $ | 11,377,126 | ||||
|
Future maturities of long-term debt as of December 31, 2020 are as follows: |
|
2021 |
$ | 690,667 | ||
|
2022 |
13,106,057 | |||
|
Total long-term debt |
$ | 13,796,724 |
The Company has a loan agreement with HSBC which is secured by a mortgage against our Central Islip, NY Headquarters. The loan is payable in 120 consecutive equal monthly installments of $25,000 in principal plus interest and a final balloon payment upon maturity in March 2022. The balances as of December 31, 2020 and December 31, 2019 were approximately $2.1 million and $2.4 million respectively. Interest accrues on the Loan, at our option, at the variable rate of LIBOR plus 1.75% or Prime less 0.5% (1.89% and 3.49% at December 31, 2020 and 2019, respectively).
On November 30, 2017, the Company purchased the premises located at 555 North Research Place, Central Islip, NY. The purchase price of the building was $13,850,000 exclusive of closing costs. The Company’s newly formed wholly-owned subsidiary, 555 N Research Corporation (the "Assignee”) and the Islip IDA, entered into a Fee and Leasehold Mortgage and Security Agreement (the ”Loan”) with HSBC in the amount of $10,387,500, which was used to finance a portion of the purchase price to acquire the premises located at 555 North Research Place, Central Islip, New York. The Loan was evidenced by the certain Note, dated November 30, 2017 (the "Note”), by and between Assignee and the Bank, and secured by a certain Fee and Leasehold Mortgage and Security Agreement (the "Mortgage”), dated November 30, 2017, as well as a collateral Assignment of Leases and Rents.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
Note 7 – Long-term Debt (continued)
The Note is payable in 60 consecutive equal monthly installments of $62,481 including interest and a final balloon payment upon maturity in December 2022. The balance outstanding as of December 31, 2020 and December 31, 2019 were approximately $9.3 million and $9.7 million respectively. The Note bears interest for each Interest Period (as defined in the Note), at the fixed rate of 3.9148%. As a condition of the Bank making the Loan, the Company was required to guaranty Assignee’s obligations under the Loan pursuant that certain Unlimited Guaranty, dated November 30, 2017 (the "Guaranty”).
On August 5, 2019, the Company entered into a Mortgage Modification Agreement which replaced the former covenant with a Minimum Liquid Assets ("MLC”) covenant, and on October 22, 2020, the Company entered into a Second Mortgage Modification Agreement modifying certain MLC balances. The Company is in compliance with its financial covenant under the mortgage at December 31, 2020.
On April 21, 2020, the Company entered into a loan agreement (the "Loan Agreement”) with HSBC Bank USA, National Association pursuant to which the Company was granted a loan in the principal amount of $2,415,970, pursuant to the Paycheck Protection Program (the "PPP”) under Division A, Title I of the CARES Act, which was enacted by the United States Congress on March 27, 2020.
The PPP loan, the obligation of which is represented by a note issued by the Company, matures on April 21, 2022 and bears interest at a rate of 1% per annum. The note may be prepaid by the Company at any time prior to maturity with no prepayment penalties. Under the terms of the PPP, all or a portion of the Loan may be forgiven, based upon payments made in the first twenty-four weeks following receipt of the proceeds, related to payroll costs, continue group health care benefits, utilities and mortgage interest on other debt obligations incurred before February 15, 2020.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
Note 8 – Earnings per Share
The calculation of basic and diluted weighted average common shares outstanding is as follows:
|
2020 |
2019 |
|||||||
|
Weighted average common shares outstanding basic earnings per share |
6,640,272 | 6,562,141 | ||||||
|
Effect of potential common share issuance: |
||||||||
|
Stock options |
- | - | ||||||
|
Weighted average common shares outstanding |
||||||||
|
Diluted earnings per share |
6,640,272 | 6,562,141 | ||||||
At December 31, 2020, stock options to purchase 417,000 shares of common stock were outstanding and 377,000 were exercisable. Stock options to purchase 432,930 shares of common stock were outstanding and 307,930 were exercisable at December 31, 2019. At December 31, 2020 and 2019, respectively, 417,000 and 307,930, stock options were not included in the computation of diluted earnings per share because their effect was antidilutive.
Note 9 – Income Taxes
At December 31, 2020, the Company had approximately $1,719,598 of federal research and development tax credits. If not utilized, the research and development tax credits expire from 2028-2040. For the year ended December 31, 2020 and 2019, the Company has provided a full valuation allowance against all of the net deferred tax assets in the amount of $3,381,133 and $2,497,414, respectively. This was based on management’s assessment, including the last two years of operating losses, that it is more likely than not that the net deferred tax assets may not be realized in the future.
On March 27, 2020, the Coronavirus Aid, Relief and Economic Security Act (the "CARES Act”) was enacted by the United States Congress. As a result of the enactment of the CARES Act, net operating losses ("NOL’s”) generated in 2018-2020 can now be carried back for five years and resulted in the Company recognizing approximately $1.5 million of a tax benefit, of which $.7 million is a receivable at December 31, 2020. We continue to evaluate for potential utilization of the Company’s deferred tax asset, which has been fully reserved for, on a quarterly basis, reviewing our economic models, including projections and timing of orders, the commencement of operations of the CVD Materials segment and cost containment measures.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
Note 9 – Income Taxes (continued)
The expense/(benefit) for income taxes includes the following:
|
2020 |
2019 |
|||||||
|
Current: |
||||||||
|
Federal |
$ | (1,528,305 | ) | $ | - | |||
|
State |
950 | (11,506 | ) | |||||
|
Total current tax provision |
(1,527,355 | ) | (11,506 | ) | ||||
|
Deferred: |
||||||||
|
Federal |
- | 1,425,414 | ||||||
|
State |
--- | --- | ||||||
|
Total deferred tax provision |
- | 1,425,414 | ||||||
|
Income tax expense / (benefit) |
$ | (1,527,355 | ) | $ | 1,413,908 | |||
The tax effects of temporary differences giving rise to significant portions of the net deferred taxes are as follows:
|
2020 |
2019 |
|||||||
|
Deferred income tax assets: |
||||||||
|
Allowance for doubtful accounts |
$ | 35,442 | $ | 5,293 | ||||
|
Inventory capitalization |
6,969 | 7,107 | ||||||
|
Impairment Charge |
712,683 | - | ||||||
|
Research & development tax credits |
1,719,598 | 588,096 | ||||||
|
Compensation costs |
211,363 | 202,287 | ||||||
|
Vacation accrual |
89,626 | 142,230 | ||||||
|
Interest expense carryforward |
223,768 | 144,340 | ||||||
|
Net operating loss carryforward |
925,912 | 1,798,315 | ||||||
|
Other items |
12,248 | 52,232 | ||||||
|
Total deferred tax asset |
3,937,609 | 2,939,900 | ||||||
|
Deferred incomes tax liability: |
||||||||
|
Property and equipment - tax over book depreciation |
(556,476 | ) | (442,486 | ) | ||||
|
Less valuation allowance |
(3,381,133 | ) | (2,497,414 | |||||
|
Net long-term deferred tax asset |
$ | - | $ | - | ||||
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
Note 9 – Income Taxes (continued)
The reconciliation of the federal statutory income tax rate to our effective tax rate is as follows:
|
2020 |
2019 |
|||||||
|
Expected provision at federal statutory tax rate (21%) |
$ | (1,596,485 | ) | $ | (1,031,888 | ) | ||
|
Provision for valuation allowance |
883,796 | 2,497,414 | ||||||
|
Foreign tax loss |
70,492 | 57,856 | ||||||
|
Net operating loss carryback |
(1,527,355 | ) | - | |||||
|
State taxes, net of federal benefit |
(36,832 | ) | (11,506 | ) | ||||
|
Federal research & development credit |
(64,266 | ) | (174,416 | ) | ||||
|
Other permanent differences |
743,295 | 76,448 | ||||||
|
Income (benefit) / tax expense |
$ | (1,527,355 | ) | $ | 1,413,908 | |||
The Company’s foreign subsidiary, CVD Tantaline ApS incurred a loss of approximately $336,000, which would provide a $74,000 deferred tax asset as of December 31, 2020, based on the standard corporate tax rate of 22% in Denmark. For the year ended December 31, 2019 the Company had a loss of $276,000 which would provide a $61,000 deferred tax asset. However, sufficient uncertainty exists as to the realizability of these assets such that a full valuation allowance has been necessary.
Note 10 – Stockholders’ equity
2001 Non-Qualified Stock Option Plan
In November 2006, the Company registered a non-qualified stock option plan that the shareholders had approved in July 2001, covering key employees, officers, directors and other persons that may be considered as service providers to the Company. Options were awarded by the Board of Directors or by a committee appointed by the Board. Under the plan, an aggregate of 300,000 shares of Company common stock, $.01 par value, were reserved for issuance or transfer upon the exercise of options which were granted. Unless otherwise provided in the option agreement, options granted under the plan would vest over a four-year period commencing one year from the anniversary date of the grant. The stock option plan expired on July 22, 2011. As of December 31, 2020 there were 7,000 options outstanding under this plan.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
Note 10 – Stockholders’ equity (continued)
2007 Share Incentive Plan
On December 12, 2007, shareholders approved the Company’s 2007 Share Incentive Plan ("Incentive Plan”), in connection therewith, 750,000 shares of the Company’s common stock are reserved for issuance pursuant to options or restricted stock that may be granted under the Share Incentive Plan through December 12, 2017. The Plan expired in December, 2017. As of December 31, 2020 there were 345,000 options outstanding under this plan.
2016 Share Incentive Plan
On December 9, 2016, shareholders approved the Company’s 2016 Share Incentive Plan ("2016 Incentive Plan”), in connection therewith 750,000 shares of the Company’s common stock are reserved for issuance pursuant to options or restricted stock that may be granted under the 2016 Incentive Plan through December 9, 2026. As of December 31, 2020, there were 65,000 options outstanding under this plan.
The purchase price of the common stock under each option plan shall be determined by the Committee, provided, however, that such purchase price shall not be less than the fair market value of the shares on the date such option is granted. The stock options generally expire seven to ten years after the date of grant. The Company recorded stock-based compensation of $255,000 and $572,000 for the years ended December 31, 2020 and 2019, respectively.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
Note 10 – Stockholders’ equity (continued)
A summary of the stock option activity related to the 2001 Stock Option Plans, the 2007 Share Incentive Plan and the 2016 Share Incentive Plan for the period from January 1, 2019 through December 31, 2020 is as follows:
|
2001 Non-Qualified Stock Option Plan |
||||||||||||||||||||||||
|
Beginning |
Granted |
Exercised |
Canceled |
Ending |
||||||||||||||||||||
|
Balance |
During |
During |
During |
Balance |
||||||||||||||||||||
|
Outstanding |
Period |
Period |
Period |
Outstanding |
Exercisable |
|||||||||||||||||||
|
Year ended December 31, 2019 |
||||||||||||||||||||||||
|
Number of shares |
22,930 | - | - | - | 22,930 | 22,930 | ||||||||||||||||||
|
Weighted average exercise price per share |
$ | 5.36 | - | - | - | $ | 5.36 | $ | 5.36 | |||||||||||||||
|
Year ended December 31, 2020 |
||||||||||||||||||||||||
|
Number of shares |
22,930 | - | - | (15,930 | ) | 7,000 | 7,000 | |||||||||||||||||
|
Weighted average exercise price per share |
$ | 5.36 | - | - | $ | 4.25 | $ | 7.90 | $ | 7.90 | ||||||||||||||
|
2007 Share Incentive Plan |
|
Beginning |
Granted |
Exercised |
Canceled |
Ending |
||||||||||||||||||||
|
Balance |
During |
During |
During |
Balance |
||||||||||||||||||||
|
Outstanding |
Period |
Period |
Period |
Outstanding |
Exercisable |
|||||||||||||||||||
|
Year ended December 31, 2019 |
||||||||||||||||||||||||
|
Number of shares |
365,000 | - | - | (20,000 | ) | 345,000 | 285,000 | |||||||||||||||||
|
Weighted average exercise price per share |
$ | 12.35 | - | - | $ | 11.61 | $ | 12.33 | $ | 12.76 | ||||||||||||||
|
Year ended December 31, 2020 |
||||||||||||||||||||||||
|
Number of shares |
345,000 | - | - | - | 345,000 | 305,000 | ||||||||||||||||||
|
Weighted average exercise price per share |
$ | 12.33 | - | - | - | $ | 12.33 | $ | 12.60 | |||||||||||||||
|
2016 Share Incentive Plan |
|
Beginning |
Granted |
Exercised |
Canceled |
Ending |
||||||||||||||||||||
|
Balance |
During |
During |
During |
Balance |
||||||||||||||||||||
|
Outstanding |
Period |
Period |
Period |
Outstanding |
Exercisable |
|||||||||||||||||||
|
Year ended December 31, 2019 |
||||||||||||||||||||||||
|
Number of shares |
20,000 | 60,000 | - | (15,000 | ) | 65,000 | - | |||||||||||||||||
|
Weighted average exercise price per share |
- | $ | 5.00 | - | $ | 5.00 | $ | 5.94 | - | |||||||||||||||
|
Year ended December 31, 2020 |
||||||||||||||||||||||||
|
Number of shares |
65,000 | - | - | - | 65,000 | - | ||||||||||||||||||
|
Weighted average exercise price per share |
$ | 5.94 | - | - | - | $ | 5.94 | - | ||||||||||||||||
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
Note 10 – Stockholders’ equity (continued)
The Company has 417,000 of outstanding stock options under the three plans at December 31, 2020.
The following table summarizes information about the outstanding and exercisable options at December 31, 2020.
|
Options Outstanding |
Options Exercisable |
|||||||||||||||||||||||||||||||
|
Weighted |
Weighted |
Weighted |
||||||||||||||||||||||||||||||
|
Average |
Average |
Average |
||||||||||||||||||||||||||||||
|
Exercise |
Number |
Remaining |
Exercise |
Intrinsic |
Number |
Exercise |
Intrinsic |
|||||||||||||||||||||||||
|
Price Range |
Outstanding |
Contractual |
Price |
Value |
Exercisable |
Price |
Value |
|||||||||||||||||||||||||
| $4.00 | - | 7.00 | 45,000 | .0 | $ | 5.00 | $ | 0 | 45,000 | $ | 5.00 | $ | 0 | |||||||||||||||||||
| $7.01 | - | 10.00 | 27,000 | .0 | $ | 8.03 | $ | 0 | 27,000 | $ | 8.03 | $ | 0 | |||||||||||||||||||
| $10.01 | - | 12.00 | 220,000 | .3 | $ | 10.81 | $ | 0 | 180,000 | $ | 11.05 | $ | 0 | |||||||||||||||||||
| $12.01 | - | 15.00 | 125,000 | 1.5 | $ | 15.00 | $ | 0 | 125,000 | $ | 15.00 | $ | 0 | |||||||||||||||||||
No options were exercised for the year ended December 31, 2020 and 2019. As of December 31, 2020, there was $12,000 of unrecognized compensation costs related to stock options expected to be recognized over a weighted average period of 1.00 years
Restricted Stock Awards
The following table summarizes restricted stock awards for the years ended December 31, 2020 and 2019:
|
Weighted |
||||||||
|
Average Grant |
||||||||
|
Shares of |
Date Fair |
|||||||
|
Restricted Stock |
Value |
|||||||
|
Unvested outstanding at December 31, 2018 |
0 | $ | 0 | |||||
|
Granted |
37,300 | $ | 3.99 | |||||
|
Vested |
(35,025 | ) | $ | 3.99 | ||||
|
Forfeited/Cancelled |
(2,275 | ) | $ | 3.99 | ||||
|
Unvested outstanding at December 31, 2019 |
0 | $ | 0 | |||||
|
Granted |
30,200 | $ | 3.74 | |||||
|
Vested |
(30,200 | ) | $ | 3.74 | ||||
|
Forfeited/Cancelled |
- | - | ||||||
|
Unvested outstanding at December 31, 2020 |
0 | $ | 0 | |||||
The total fair value of shares of restricted stock awards vested for the years ended December 31, 2020 and 2019 was approximately $113,000 and $140,000 respectively. The fair value of the outstanding restricted stock awards is recorded as stock compensation expense over the vesting period.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
Note 10 – Stockholders’ equity (continued)
Restricted Stock Units
The following table summarizes restricted stock units for the years ended December 31, 2020 and December 31, 2019:
|
Weighted |
||||||||
|
Shares of |
Average Grant |
|||||||
|
Restricted |
Date Fair |
|||||||
|
Stock Units |
Value |
|||||||
|
Unvested outstanding at December 31, 2018 |
46,063 | $ | 8.25 | |||||
|
Granted |
10,500 | $ | 4.15 | |||||
|
Vested |
(33,988 | ) | $ | 9.08 | ||||
|
Forfeited/Cancelled |
(- | ) | $ | - - | ||||
|
Unvested outstanding at December 31, 2019 |
22,575 | $ | 4.66 | |||||
|
Granted |
- | - | ||||||
|
Vested |
(12,825 | ) | $ | 8.81 | ||||
|
Forfeited/Cancelled |
(1,000 | ) | $ | 5.45 - | ||||
|
Unvested outstanding at December 31, 2020 |
8,750 | $ | 5.00 | |||||
The total fair value of vested restricted stock units was $91,000 and $309,000 respectively for the years ended December 31, 2020 and 2019.
The fair value of the outstanding restricted stock units will be recorded as stock compensation expense over the vesting period. As of December 31, 2020, there was $25,000 of total unrecognized compensation costs related to restricted stock units, which is expected to be recognized over a weighted-average period of 1.0 years.
During the years ended December 31, 2020 and 2019, the Company recorded into selling and general administrative expense approximately $255,000 and $572,000 of stock-based compensation expense for the cost of employee and director services received in exchange for equity instruments based on the grant-date fair value of those instruments in accordance with the provisions of ASC 718.
Note 11 – Defined Contribution Plan
The Company maintains a 401(k) Plan for the benefit of all eligible employees. All employees as of the effective date of the 401(k) Plan became eligible. An employee is eligible to become a participant after three months of continuous service.
Participants may elect to contribute from their compensation any amount up to the maximum deferral allowed by the Internal Revenue Code. Employer contributions are optional. No discretionary employer contribution has been made for 2020 and 2019.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
Note 12 – Segment Reporting
The Company operates through three segments, CVD, SDC and CVD Materials. The CVD segment is utilized for silicon, silicon germanium, silicon carbide and gallium arsenide processes. SDC is the Company’s ultra-high purity manufacturing division in Saugerties, New York. The accounting policies of CVD and SDC are the same as those described in the summary of significant accounting policies (see Note 2). The Company evaluates performance based on several factors, of which the primary financial measure is earnings before taxes. Included in the CVD Materials segment are our wholly owned subsidiaries, CVD Tantaline Aps and CVD MesoScribe Technologies Corporation.
The following table presents certain information regarding the Company’s segments as of December 31, 2020 and December 31, 2019 and for the years then ended:
|
2020 |
||||||||||||||||||||||||
|
(In thousands) |
CVD |
SDC |
Materials |
Corporate |
Eliminations * |
Consolidated |
||||||||||||||||||
|
Assets |
$ | 31,284 | $ | 6,068 | $ | 3,593 | $ | (9 | ) | $ | 40,936 | |||||||||||||
|
Revenue |
$ | 10,385 | $ | 4,429 | $ | 2,556 | $ | (450 | ) | $ | 16,920 | |||||||||||||
|
Operating (loss)/income |
(863 | ) | 473 | (4,283 | ) | (3,151 | ) | (7,824 | ) | |||||||||||||||
|
Pretax (loss)/income |
(878 | ) | 481 | (4,054 | ) | (3,151 | ) | (7,602 | ) | |||||||||||||||
|
2019 |
||||||||||||||||||||||||
|
(In thousands) |
CVD |
SDC |
Materials |
Corporate |
Eliminations * |
Consolidated |
||||||||||||||||||
|
Assets-revised |
$ | 34,374 | $ | 6,003 | $ | 6,355 | $ | (9 | ) | $ | 46,723 | |||||||||||||
|
Revenue |
$ | 14,065 | $ | 4,498 | $ | 1,786 | $ | (702 | ) | $ | 19,647 | |||||||||||||
|
Operating (loss)/income |
(1,811 | ) | 696 | (537 | ) | (3,333 | ) | (4,985 | ) | |||||||||||||||
|
Pretax (loss)/income |
(1,782 | ) | 719 | (518 | ) | (3,333 | ) | (4,914 | ) | |||||||||||||||
|
*All elimination entries represent intersegment transactions eliminated in consolidation for external reporting. |
|
2020 Materials segment includes an impairment charge of $3.6 million related to the Tantaline product line |
NOTE 13: Significant Events- COVID-19
The Company has been actively monitoring the coronavirus (COVID-19) outbreak and resulting pandemic and its impact on both the global economic and operating environment and specifically on its impact to the Company, its employees, its operations and its financial condition. In March 2020, the World Health Organization recognized the COVID-19 outbreak as a pandemic based on the global spread of the disease, the severity of illnesses it causes and its effects on society. In response to the COVID-19 outbreak, the governments of many countries, states, cities and other geographic regions have taken preventative or protective actions, such as imposing restrictions on travel and business operations, including complete or partial government shutdowns of many schools and businesses, including our Company, and advising or requiring individuals to limit or forego their time outside of their homes. Accordingly, the COVID-19 outbreak has severely restricted the level of economic activity in many countries, including the United States, and continues to materially and adversely impact global economic activity. In particular, the aerospace sector, for which we rely on a significant part of our business, has been faced with significant reductions to its business due to lack of air travel. The Company’s new order levels during the year ended December 31, 2020 and into the first quarter of 2021 have seen substantial reductions which have materially and adversely affected revenues commencing in our second quarter of 2020, and is anticipated to continue into 2021. While the financial results for the Company’s first quarter of 2020 reflected the initial impact of COVID-19, and the twelve months ended December 31, 2020 reflected a substantial adverse effect, we are unable to predict the extent of the impact the pandemic will have on our financial position and operating results for 2021 due to numerous uncertainties, but the impact could be material during any future period affected either directly or indirectly by this pandemic. The Company intends to continue to evaluate the various government sponsored plans and programs put in place in response to the COVID-19 pandemic and further plans to take advantage of any such government benefits reasonably available to it. Moreover, the Company will continue to monitor developments in that area as new government initiatives are passed.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
Note 14 – Subsequent Events
In January 2021, the Board of Directors made a decision to implement a change in direction and new leadership to evaluate the business strategy and operations. As such, they appointed Emmanuel Lakios as President and Chief Executive Officer (previously our Vice-President- Sales and Marketing). A decision was made based on continued losses and significant investments required to continue in the Materials business that our primary focus should be on the core equipment business and that the Materials Business strategy should be revised, with some of its current elements potentially minimized or ceased. Based upon an analysis, including forecasted continued losses and negative cash flows for the Tantaline product line, we have implemented plans to eliminate further investment in our Tantaline product line. In addition, we have recorded an impairment charge of $3.6 million during the fourth quarter and year ended December 31, 2020. In addition, we continue to monitor our costs and will take actions to mitigate expenses in the future.
In order to increase our liquidity and to provide necessary working capital to support our on-going business and operations, we have decided to sell the 555 Building in February 2021. We have determined the 555 Building is not needed for present and future business operations. We have concluded that any remaining elements of the Materials Business can be consolidated into the 355 Building, which we believe can accommodate any needs for our growth for the foreseeable future.
On March 29, 2021, the Company entered into an agreement with Steel K, LLC for the sale of its 555 Building. The purchase price is $24,360,000, and the closing of the sale is subject to the satisfaction or waiver of certain conditions to closing or contingencies. A portion of the sale proceeds would be used to satisfy the existing mortgage debt on the 555 Building in the approximate amount of $9.3 million at December 31, 2020, and for various costs related to the sale closing in an amount to be determined. Any excess proceeds will be used for general working capital purposes.
Exhibit 10.26



















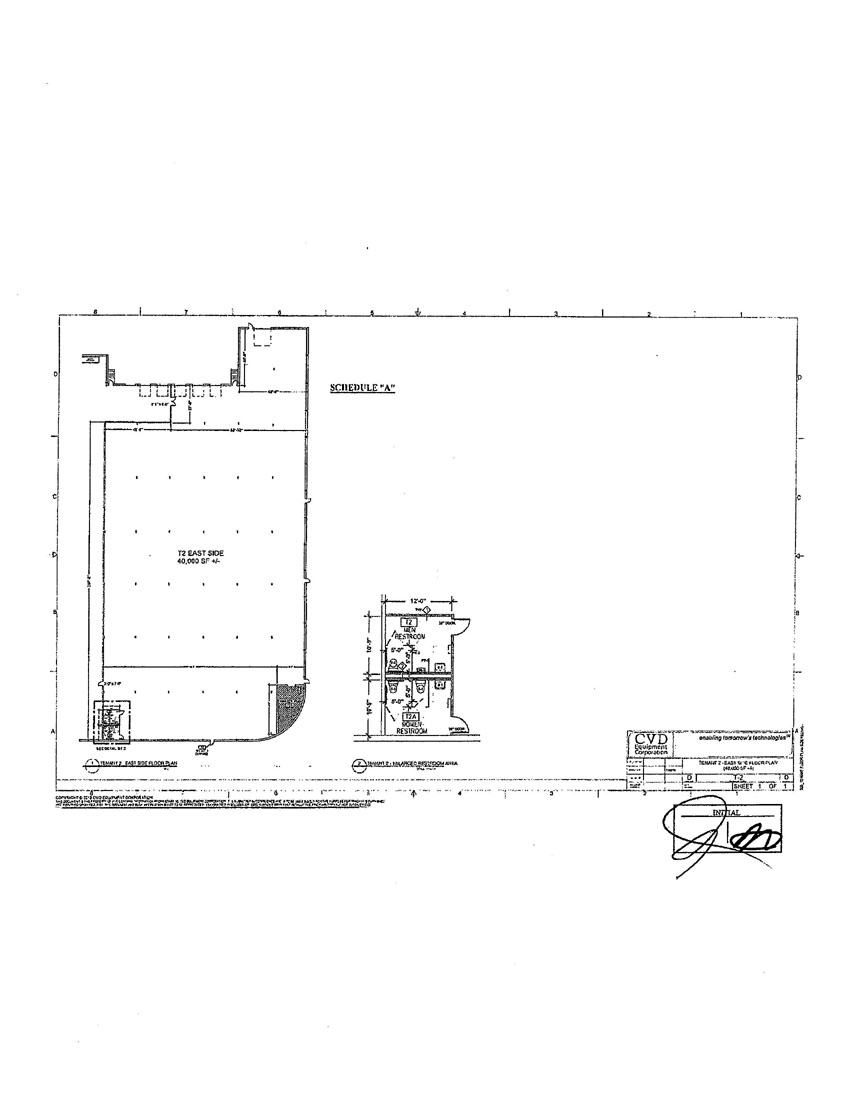



Exhibit 23.1
INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM’S CONSENT
We consent to the incorporation by reference in the Registration Statement of CVD Equipment Corporation on Form S-8 (File No. 333-138903, 333-153186, and 333-217439) of our report dated March 31, 2021, with respect to our audit of the consolidated financial statements of CVD Equipment Corporation and Subsidiaries as of December 31, 2020 and 2019 and for each of the two years in the period ended December 31, 2020, which report is included in this Annual Report on Form 10-K of CVD Equipment Corporation for the year ended December 31, 2020.
/s/ Marcum llp
Marcum llp
Melville, NY
March 31, 2021
Exhibit 31.1
Certification of Principal Executive Officer
Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
I, Emmanuel Lakios, certify that:
|
1. |
I have reviewed this annual report on Form 10-K of CVD Equipment Corporation; |
2. Based upon my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3 Based upon my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4. The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f) for the registrant and have:
(a) Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
(b) Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
(c) Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
(d) Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5. The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
(a) All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial data; and
(b) Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
|
Dated: March 31, 2021 |
/s/ Emmanuel Lakios |
|
President, Chief Executive Officer |
Exhibit 31.2
Certification of Principal Financial Officer
Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
I, Thomas McNeill, certify that:
1. I have reviewed this annual report on Form 10-K of CVD Equipment Corporation;
2. Based upon my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3 Based upon my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4. The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act rules 13a-15(f) and 15d-15(f) for the registrant and have:
(a) Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
(b) Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
(c) Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
(d) Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5. The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
(a) All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial data; and
(b) Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
|
Dated: March 31, 2021 |
/s/ Thomas McNeill |
|
Chief Financial Officer |
Exhibit 32.1
Certification of Principal Executive Officer
Pursuant to 18 U.S.C. Section 1350, as adopted pursuant to
Section 906 of the Sarbanes-Oxley Act of 2002
I, Emmanuel Lakios, President and Chief Executive Officer of CVD Equipment Corporation, hereby certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that to my knowledge, the annual report on Form 10-K for the period ending December 31, 2020 of CVD Equipment Corporation (the "Form 10-K”) fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934 and the information contained in the Form 10-K fairly presents, in all material respects, the financial condition and results of operations of CVD Equipment Corporation.
|
Dated: March 31, 2021 |
/s/ Emmanuel Lakios |
|
Emmanuel Lakios |
|
|
Chief Executive Officer |
|
|
(Principal Executive Officer) |
Exhibit 32.2
Certification of Principal Financial Officer
Pursuant to 18 U.S.C. Section 1350, as adopted pursuant to
Section 906 of the Sarbanes-Oxley Act of 2002
I, Thomas McNeill, Chief Financial Officer of CVD Equipment Corporation, hereby certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that to my knowledge, the annual report on Form 10-K for the period ending December 31, 2020 of CVD Equipment Corporation (the "Form 10-K”) fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934 and the information contained in the Form 10-K fairly presents, in all material respects, the financial condition and results of operations of CVD Equipment Corporation.
|
Dated: March 31, 2021 |
/s/ Thomas McNeill |
|
Thomas McNeill |
|
|
Chief Financial Officer |
|
|
(Principal Financial Officer) |